Phesi’s AI-driven Trial Accelerator platform contains over 100 million patients
Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development

Rethinking Clinical Trials
OCTOBER 2, 2023
The Patient-Centered Outcomes Core has developed a new tool kit to provide resources to support the capture of patient-reported outcome (PRO) measures in diverse study populations. The post October 2, 2023: Patient-Centered Outcomes Core Develops Tool Kit to Promote Health Equity in PROs appeared first on Rethinking Clinical Trials.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Discovery World
AUGUST 4, 2022
It may seem counterintuitive to spend time and money on planning for containment and delivery systems for a drug in the earliest stages of discovery when the chances of that molecule making it to market are still quite low. However, starting early can greatly improve the efficiency and speed of the development process. Never too early.
Pharmaceutical Technology
DECEMBER 12, 2022
Gilead company Kite has entered an international strategic partnership with Arcellx for the joint development and commercialisation of the latter’s T-cell therapy, CART-ddBCMA, to treat relapsed or refractory multiple myeloma patients. Additionally, Kite will make other potential payments to Arcellx.
Pharmaceutical Technology
OCTOBER 6, 2022
from the US National Institutes of Health (NIH) unit National Institute of Allergy and Infectious Diseases (NIAID) to develop a prophylactic intranasal vaccine against Neisseria gonorrhoeae (NG). Leveraging its outer membrane vesicles (OMV) platform technology, Intravacc will develop the vaccine.
XTalks
APRIL 25, 2024
Now we recognize that over the last ten years or so there have been major developments in pharmacotherapy for the management of different etiologies of heart failure,” says Dr. Nicholas Alp, MD, PhD, FACC, FRCP, Vice President of the Medical Department at Medpace. Survival rates were also impressive, exceeding 90 percent at one year.

Camargo
OCTOBER 14, 2021
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Continued development of the use of complex innovative trial designs. Continue development of the use of Real-World Evidence.

pharmaphorum
JANUARY 14, 2022
The European Commission, EMA and national regulators within the EU have launched an initiative to change the way clinical trials are designed and run in order to position the bloc as an international “focal point” for clinical research.

XTalks
MAY 4, 2023
When a person with celiac disease eats something that contains gluten, their immune system attacks their small intestine, damaging the lining and interfering with the absorption of nutrients from food. However, there are ongoing clinical trials for celiac disease to investigate potential new treatments.
Pharmaceutical Technology
MAY 11, 2023
The vaccine, developed by BioNTech, led to half of the patients with pancreatic cancer in the Phase I trial remaining cancer-free 18 months later. A global randomised follow-up trial is planned. In 2016, BioNTech and Genentech, a part of Roche signed an agreement to develop personalised mRNA therapies in oncology.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Still, navigating such patent issues during drug development can be difficult, and Camargo can assist in finding a safe course. Ken Phelps.

Drug Discovery World
DECEMBER 6, 2022
As a result of clear US Food and Drug Administration (FDA) guidelines, the leading vaccine development programmes for Covid-19 were all remarkably similar. Indeed, few randomised trials of NPIs have been undertaken. Quasi-experimental and other randomised trial designs . Pre-specification of effect size .

Camargo
DECEMBER 13, 2021
The development of biological products (or biologics) represents a major advancement in modern medicine, enabling the treatment of patients with many illnesses where no other therapeutics were previously available. Regulatory Considerations for Biologics. Section 351(a) is the traditional pathway for approving biologics under the PHS Act.

Pharmaceutical Technology
APRIL 14, 2023
Ghana’s Food and Drug Authority (FDA) has approved R21/Matrix-M malaria vaccine in children aged 5 to 36 months, marking the first regulatory clearance for the University of Oxford-developed vaccine in any country in the world. The WHO, which has already recommended GSK’s RTS,S/AS01 malaria vaccine , is yet to recommend Oxford’s R21 vaccine.

pharmaphorum
DECEMBER 4, 2022
Clinical trials of a wireless brain chip developed by Elon Muck’s Neuralink company will be tested in human volunteers within the next six months – and Musk himself says he intends to have one implanted for a future demo event. The post Elon Musk’s Neuralink brain interface chip set for human trials appeared first on.

Pfizer
AUGUST 3, 2022
Pfizer to Discontinue Development Program for PF-07265803 for LMNA-Related Dilated Cardiomyopathy. Pfizer to Discontinue Development Program for PF-07265803 for LMNA-Related Dilated Cardiomyopathy. Decision follows results of interim futility analysis which indicate Phase 3 REALM-DCM trial unlikely to meet primary endpoint.
Drug Discovery World
OCTOBER 31, 2023
UK regulatory authorities have approved the first trial of a gene therapy for young children with Hunter syndrome. The drug was developed over eight years by Brian Bigger, Professor of Cell and Gene Therapy at The University of Manchester.

Camargo
JUNE 4, 2021
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. In the News: May 2021 Regulatory and Development Updates. In the News: April 2021 Regulatory and Development Updates.

Drug Discovery World
MARCH 28, 2023
SolasCure’s first investigational product, Aurase Wound Gel, a hydrogel containing an enzyme cloned from medical maggots which aims to accelerate wound debridement, is due to enter further Phase II efficacy-supporting trials. funding to develop Aurase Wound Gel appeared first on Drug Discovery World (DDW).

XTalks
NOVEMBER 29, 2022
Xtalks interviewed Devon Adams to learn more about his work related to decentralized oncology trials as a senior analyst for legislative policy specific to clinical trials at the ACS CAN. Read on to learn more! Over 1,100 cancer patients and cancer survivors responded.

Pharmaceutical Technology
APRIL 28, 2023
It has also been approved by the FDA for otitis media prevention in infants and children aged between six weeks and five years caused by the original seven serotypes contained in PREVNAR. The latest approval is based on data obtained from the Phase II and Phase III trials of PREVNAR 20 for paediatric indication.

pharmaphorum
NOVEMBER 28, 2021
An investigator-led trial of Jazz Pharma’s cannabis extract-based drug Sativex in glioblastoma – an aggressive form of brain cancer – will get underway in the UK next year. billion takeover of Sativex’ developer GW Pharma earlier this year. Jazz acquired rights to Sativex when it completed its $7.2

Pharmaceutical Technology
OCTOBER 31, 2022
After decades of setbacks, the respiratory syncytial virus (RSV) vaccine field has bounced back with positive Phase III trial results in older adults. There are currently five players in the race, with vaccines in Phase III of development from GlaxoSmithKline (GSK) , Pfizer , Johnson & Johnson , Moderna and Bavarian Nordic.

Worldwide Clinical Trials
FEBRUARY 21, 2024
During clinical development, new chemical entities (NCEs) require an absorption, metabolism, and excretion (AME) study. Regardless of the formulation, the entire dose must be administered to each subject, and the dosing containers must be checked for residual radioactivity.

XTalks
APRIL 24, 2023
Rare disease clinical trials are complex due to the additional scientific, medical, operational and regulatory requirements of newly emerging advanced therapies, such as gene therapy,” says Dr. Terence Eagleton, MB BS, Senior Medical Director at the global clinical research organization (CRO) Medpace.
Cloudbyz
SEPTEMBER 16, 2023
Addressing data privacy and data protection concerns when implementing ChatGPT in clinical trial operations management is crucial to maintain compliance with regulations, safeguard sensitive patient information, and build trust among stakeholders.
Drug Discovery World
AUGUST 9, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted Aleta Biotherapeutics a clinical trial authorisation (CTA) to evaluate biologic ALETA-001 in a Phase I/II clinical trial in patients with B-cell malignancies who are relapsed/refractory to CD19 CAR T cell therapy.

CTTI (Clinical Trials Transformation Initiative)
JANUARY 10, 2024
Timely, accurate, and complete registration and reporting of summary results information for applicable clinical trials on ClinicalTrials.gov allows access to current research and evidence for all partners in the clinical trials enterprise, including patients, providers, sponsors and investigators, regulators, payers, and health system leaders.
pharmaphorum
SEPTEMBER 14, 2020
AstraZeneca has resumed UK trials for its coronavirus vaccine, after the country’s medicines regulator gave the all-clear following a safety scare. A UK safety committee has concluded its investigations and recommended to the country’s Medicines and Healthcare products Regulatory Agency (MHRA) that trials are safe to resume.

Drug Discovery World
OCTOBER 19, 2022
Yapan Bio has expanded its capabilities with a new process development facility at Genome Valley, Hyderabad, India, which has enhanced its ability to support end-to-end development and manufacturing of RNA, DNA and gene therapy products starting from plasmids. .

Cloudbyz
APRIL 9, 2023
Clinical trial management involves various activities and processes that can have a significant impact on the environment and contribute to sustainability issues. Clinical trials, in particular, have a significant impact on the environment, and it is essential to address this impact to ensure a sustainable future.

Drug Discovery World
NOVEMBER 9, 2022
However, there is an increasing need to predict metabolism for other enzymes, such as human Aldehyde Oxidates (AOs), Flavin-containing Monooxygenases (FMOs), and Uridine 5’-diphosphoglucuronosyltransferases (UGTs). . The post AI development duo to advance drug-metabolising enzymes appeared first on Drug Discovery World (DDW).
Drug Discovery World
JANUARY 6, 2023
ISA103 was developed using the company’s Synthetic Long Peptide (SLP) technology, designed to fully harness and direct the body’s defense mechanisms towards fighting the disease. It contains multiple long peptides spanning the most immunogenic regions of the PRAME protein. . A total of 90 patients will be enrolled in the trial. .

Pharmaceutical Technology
SEPTEMBER 20, 2022
Unlike commercial pharmaceutical packaging, the primary consideration in clinical trial packaging is protecting the product quality and reliability for research. Finding the best clinical trial packaging services providers. Clinical trial packaging and labelling solutions. Clinical trial packaging and labelling solutions.

Pfizer
FEBRUARY 16, 2023
These study participants, representing approximately half of the total recruited participants in the trial, are being discontinued following violations of Good Clinical Practice (GCP) at certain clinical trial sites run by a third-party clinical trial site operator.
Pharmaceutical Technology
JUNE 9, 2023
VEVYE, the development name of which is CyclASol, is a cyclosporine, solubilised solution in a new, water-free excipient. CyclASol is topical anti-inflammatory and immunomodulating ophthalmic drug solution containing 0.1%
Cloudbyz
JUNE 16, 2023
Clinical trials are crucial for advancing medical research and developing innovative treatments. Effective clinical trial data archiving is essential to ensure data integrity, regulatory compliance, and seamless access. Data Complexity: Clinical trial data often comes in diverse formats, such as text, images, audio, and video.

Advarra
OCTOBER 4, 2023
When it comes to clinical development, precision, compliance, and quality assurance are paramount. For clinical development organizations, an effective CAPA serves as an essential compass, directing a path towards continuous improvement while maintaining steadfast regulatory compliance.
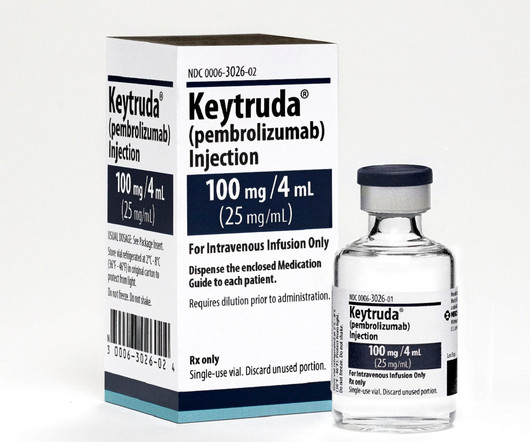
Pharmaceutical Technology
APRIL 4, 2023
KEYTRUDA is an anti-PD-1 therapy developed by Merck, while Padcev has been developed by Astellas and Seagen. The combination therapy can be used to treat la/mUC patients who do not qualify for cisplatin-containing chemotherapy. The trial was jointly conducted by Seagen and Astellas.

STAT News
SEPTEMBER 26, 2022
Here’s a scenario anyone who has done clinical research will recognize: A 32-year-old woman participating in a Phase 1 healthy-volunteer crossover clinical trial tested negative for pregnancy when she enrolled and agreed to use contraception during the course of the trial, as specified in the protocol.
pharmaphorum
FEBRUARY 24, 2022
Clinical stage pharmaceutical company Cantex Pharmaceuticals has obtained a global licence from Harvard University’s Office of Technology Development to develop the small-molecule drug azeliragon into a treatment for inflammatory lung diseases, including COVID-19. said Cantex CEO Stephen Marcus.
Drug Discovery World
OCTOBER 9, 2023
Positive Phase I trial data indicates that Reqorsa (quaratusugene ozeplasmid) has shown early efficacy for the treatment of non-small cell lung cancer (NSCLC). Developed by gene therapy company Genprex, Reqorsa is a non-viral gene therapy that leads to expression of the TUSC2 tumour suppressor gene in cancers.
pharmaphorum
AUGUST 6, 2021
Psychiatric disorders in particular represent a trial area with significantly high drop-out rates and poor patient recruitment. Despite this, new strategies devised over the last five years are beginning to reverse the trend, and both reduce stigma and increase awareness to funnel new patients into these important trials. .

Pharmaceutical Technology
SEPTEMBER 14, 2022
Pharmaceutical drug research and development (R&D) activities are capital-intensive, which makes the outsourcing of clinical dose manufacturing and marketing popular. The download contains detailed information on the providers and their services and solutions, alongside contact details to aid your purchasing or hiring decision.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content