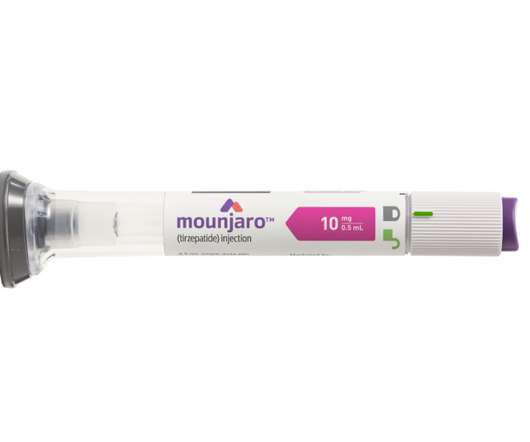
Lilly’s new drug Mounjaro (tirzepatide ) wins US FDA approval
pharmaphorum
MAY 16, 2022
GLP-1 and GIP are hormones involved in blood sugar control and Mounjaro, a first-in-class medicine that activates both GLP-1 and GIP receptors, demonstrated improved blood sugar control. The findings were made via Lilly’s SURPASS phase 3 global clinical development programme, which comprised studies ranging from 40 to 52 weeks.



















Let's personalize your content