Sourcing Blockbuster Oncology Products for Clinical Trial Supply
XTalks
JANUARY 29, 2021
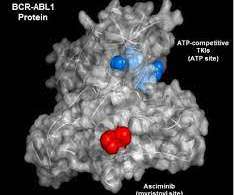
This is relevant for clinical trials as more regulators require evidence of efficacy in comparison to the standard of care, which is likely to be one of the blockbuster products. This means that sponsors are having to fork out for these blockbuster products in order to run their trials more often.




































Let's personalize your content