Konvomep Gets FDA Approval as a New Oral Liquid Formulation Option of Omeprazole
XTalks
SEPTEMBER 6, 2022
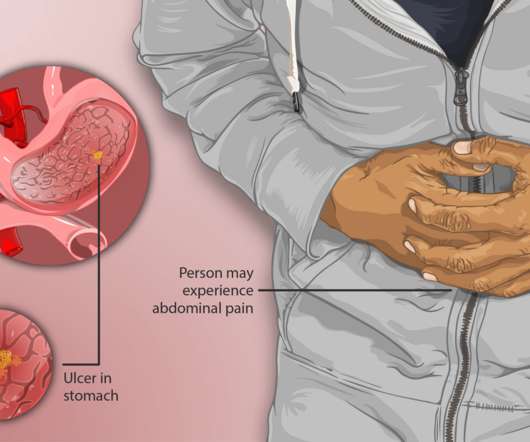
Doctors may prescribe a proton pump inhibitor (PPI), such as omeprazole, which blocks acid-producing cells to protect the gastric ulcer from acid while it heals naturally. Konvomep is contraindicated in patients with known hypersensitivity to any components of the formulation and in patients receiving rilpivirine-containing products.














Let's personalize your content