Prevention of Invasive Pneumococcal Disease in Adults 18 Years and Older Caused by 15 Serotypes
The Pharma Data
JULY 18, 2021
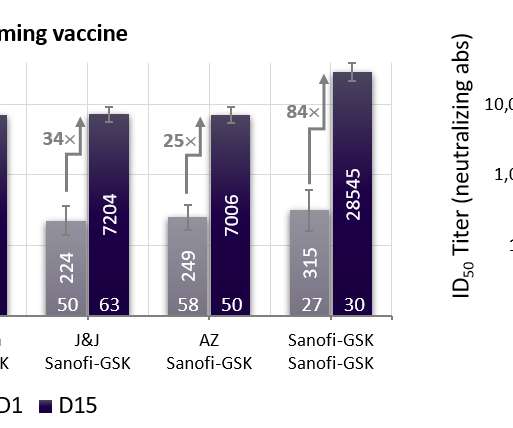
Clinical Data Supporting Approval Demonstrated Non-Inferior Immune Responses for the Serotypes Shared with PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F). VAXNEUVANCE Elicited Superior Immune Responses for Serotypes 3, 22F and 33F Compared to PCV13, Which Are Major Causes of Disease.



































Let's personalize your content