Vevye: A New Cyclosporine Solution for Dry Eye Disease
XTalks
JUNE 14, 2023
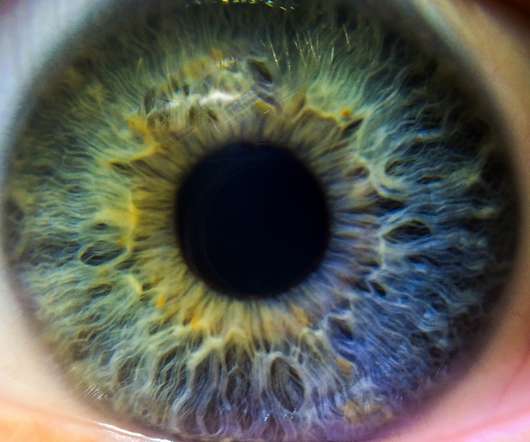
The solution of Vevye does not contain water, anti-microbial preservatives, oils or surfactants. Miebo contains perfluorohexyloctane as its active ingredient, a semifluorinated alkane that forms a protective layer over the tear film, reducing evaporation at the ocular surface. Vevye (cyclosporine ophthalmic solution) 0.1













Let's personalize your content