Drug made from pig intestine helps escape the “trap” of clot-causing immune response
Scienmag
DECEMBER 9, 2021
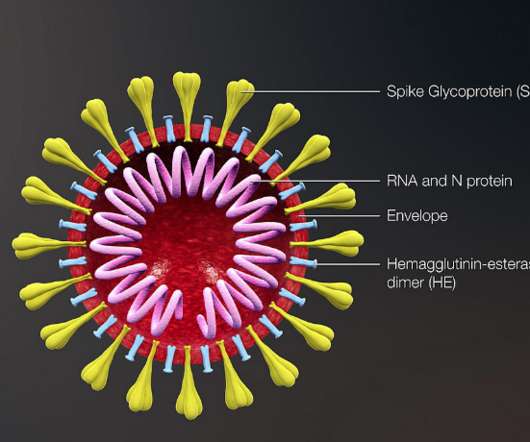
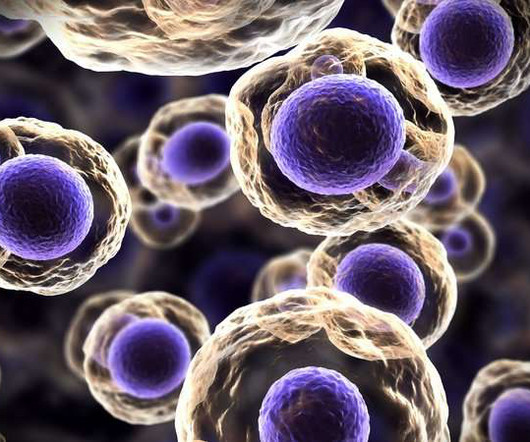
When the body attempts to fight off an infection, immune cells called neutrophils may shoot out spider web-like networks of toxic proteins to help contain the invaders.
























Let's personalize your content