Moderna’s founder launches Laronde, promising new ‘Endless RNA’ drug class
pharmaphorum
MAY 11, 2021
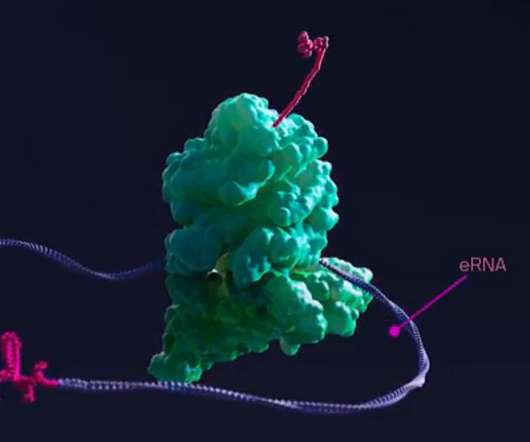
Flagship Pioneering, the VC fund run by Moderna’s co-founder Noubar Afeyan has launched a new biotech Laronde , with an ambitious plan to create a new class of drugs based on Endless RNA. Called eRNA for short, this class of medicines is programmable and can continuously express therapeutic proteins inside the body.



















Let's personalize your content