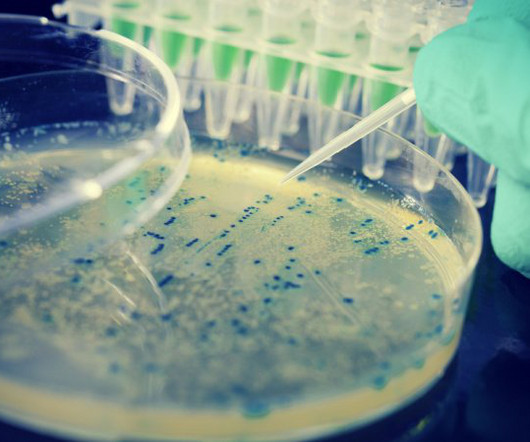
Scientists engineer safe, virus-resistant E coli for research
Drug Discovery World
MARCH 21, 2023
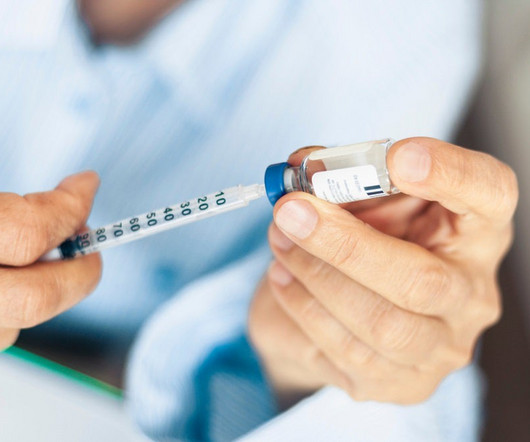
The team used two safeguard methods to prevent the bacteria and their modified genes from escaping into the wild. The work promises to reduce the threats of viral contamination when harnessing bacteria to produce medicines such as insulin as well as other useful substances, such as biofuels.










Let's personalize your content