ALS drug from Denali, Sanofi falls short in mid-stage study
Bio Pharma Dive
FEBRUARY 16, 2024
The disclosure the experimental therapy missed its trial goal comes after Sanofi had earmarked the drug for advancement into Phase 3.

Bio Pharma Dive
FEBRUARY 16, 2024
The disclosure the experimental therapy missed its trial goal comes after Sanofi had earmarked the drug for advancement into Phase 3.

Pharmaceutical Technology
FEBRUARY 16, 2024
This positive news comes after Otsuka’s Phase III Alzheimer’s agitation trial failed to meet its primary endpoint, announced earlier this week.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
FEBRUARY 16, 2024
Kelonia will receive $40 million upfront through a partnership to develop “off-the-shelf” cell therapies for cancer.

Pharmaceutical Technology
FEBRUARY 16, 2024
GSK has successfully concluded the acquisition of Aiolos Bio in a deal that could reach $1.4bn, expanding its respiratory portfolio.

Bio Pharma Dive
FEBRUARY 16, 2024
The agency will decide by June 21 whether to enable more patients with the disease to receive Elevidys, and won’t convene a group of outside experts beforehand.

Pharmaceutical Technology
FEBRUARY 16, 2024
Dragonfly Therapeutics has entered a clinical collaboration with Gilead Sciences to assess DF1001 plus Trodelvy for cancer indications.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
FEBRUARY 16, 2024
Regeneron and Sanofi's Dupixent has been approved for the treatment of chronic spontaneous urticaria (CSU) or itching in Japan.

Fierce Pharma
FEBRUARY 16, 2024
The T-cell therapy treatment class, which has transformed the treatment of certain blood cancers, has now reached the solid tumor field thanks to an FDA approval for a first-of-its-kind immunothera | The T-cell therapy treatment class, which has transformed the treatment of certain blood cancers, has now reached the solid tumor field thanks to an FDA approval for a first-of-its-kind immunotherapy developed by Iovance Biotherapeutics.

Pharmaceutical Technology
FEBRUARY 16, 2024
Despite failing its primary endpoint in the EMBARK confirmatory study, Elevidys’ efficacy supplement has gained FDA priority review.

Fierce Pharma
FEBRUARY 16, 2024
People with food allergies finally have a drug that can help prevent severe outcomes—and it’s a drug that’s been on the market for two decades. | People with food allergies finally have a drug that can help prevent severe outcomes—and it’s a drug that’s been on the market for two decades. The FDA has blessed Roche and Novartis’ Xolair as the first medicine to reduce allergic reactions that can occur with accidental exposure to certain foods.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
FEBRUARY 16, 2024
The US FDA has granted orphan drug designation to Ionis Pharmaceuticals’ olezarsen for familial chylomicronemia syndrome (FCS).

Fierce Pharma
FEBRUARY 16, 2024
Gene editing’s therapeutic application has transitioned from hypothetical to reality, marked by the recent approval of a CRISPR-based therapy for sickle cell and beta thalassemia. | This week on "The Top Line," Max Bayer from Fierce Biotech explores the future of gene editing in an interview with the CEO of Verve Therapeutics.

Pharmaceutical Technology
FEBRUARY 16, 2024
Xyphos Biosciences has entered an agreement with Kelonia Therapeutics to develop immuno-oncology therapeutics for $875m.
pharmaphorum
FEBRUARY 16, 2024
CAR-T therapies can achieve remarkable efficacy in the treatment of haematological cancers, but the risk of side effects means that the cell infusions are almost always administered to inpatients under close supervision in clinics.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
FEBRUARY 16, 2024
Though the designation is for soft tissue sarcoma, tigilanol tiglate is in clinical trials for multiple cancer types.

pharmaphorum
FEBRUARY 16, 2024
Recent biotech financings include a $245m raise for cancer blood test company Freenome, plus big rounds for Cogent Biosciences, BioAge Labs, Latigo Biotherapeutics, ProfoundBio, and Firefly Bio

Fierce Pharma
FEBRUARY 16, 2024
A Novartis subsidiary in India has become the target of a strategic review as the Swiss pharma focuses its efforts on newer medicines. | A Novartis subsidiary handling old products in India has become the target of a strategic review as the Swiss pharma focuses its efforts on newer medicines.

pharmaphorum
FEBRUARY 16, 2024
Gilead Sciences has paused enrolment in clinical trials of its CD47 drug magrolimab in solid tumours, a week after dropping it for blood cancers.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
FEBRUARY 16, 2024
While Johnson & Johnson’s consumer health spinoff Kenvue prepares to move into a swanky new headquarters in Summit, New Jersey, it appears some staffers won’t be joining the company in the tran | While Johnson & Johnson’s consumer health spinoff Kenvue prepares to move into a new headquarters in Summit, New Jersey, it appears some staffers won’t be joining the company in the transition.

pharmaphorum
FEBRUARY 16, 2024
Astellas has signed a deal with Kelonia Therapeutics that aims to push the boundaries of what can be achieved with CAR-T therapies in immuno-oncology

Pharma Times
FEBRUARY 16, 2024
Cardiac quantitative susceptibility mapping successfully visualised iron deposits in patients

pharmaphorum
FEBRUARY 16, 2024
The ABPI has released a new guide on how the industry can support clinicians with prescribing decisions. Find out more about how pharmaceutical companies can assist healthcare professionals in providing optimal patient care.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Fierce Pharma
FEBRUARY 16, 2024
Four months after the FDA rejected Sanofi and Regeneron’s Dupixent to treat chr | Four months after the FDA rejected Sanofi and Regeneron’s Dupixent to treat chronic spontaneous urticaria, Japan’s health regulators have signed off on the drug in the indication.
pharmaphorum
FEBRUARY 16, 2024
Explore the role of artificial intelligence and machine learning in drug discovery and the challenges associated with patentability and predictability. Learn how AI is revolutionising the pharmaceutical industry.

Drug Channels
FEBRUARY 16, 2024
Today’s guest post comes from Gavin Magaha, Senior Director of Value Delivery at Kalderos. Gavin discusses the problems manufacturers face in accessing and standardizing drug discount data. These data are necessary to avoid duplicate discounts and comply with regulatory requirements. To learn more about how the Inflation Reduction Act adds to challenges of drug discount programs, download Kalderos’ whitepaper: Operational Complexity and Evolving Challenges: What Drug Manufacturers Need to Know N
pharmaphorum
FEBRUARY 16, 2024
Patterns of behaviour: Using AI-based algorithms to improve outcomes for patients with atrial fibrillation Mike.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Commerce
FEBRUARY 16, 2024
The financial commitment features construction of new warehouses in Pennsylvania and North Carolina.

pharmaphorum
FEBRUARY 16, 2024
In this interview, Jonah Comstock speaks with Xavier Duportet, the founder and CEO of the biotech company, Eligo Bioscience, about the future of CRISPR gene editing at JP Morgan in 2024. Dive into the discussion to gain insights into this cutting-edge technology and its potential impact on the healthcare industry.
Pharma Times
FEBRUARY 16, 2024
NRTX-1001 is being assessed for drug-resistant mesial temporal lobe epilepsy
Drug Discovery World
FEBRUARY 16, 2024
Novartis’ Cosentyx (secukinumab) is now available in Scotland, following positive advice from the Scottish Medicines Consortium (SMC). It is licensed for adults with active moderate to severe hidradenitis suppurativa (HS) (acne inversa) who have responded inadequately to conventional systemic HS therapy. The advice applies for use in adult patients with active moderate to severe HS for whom adalimumab is contraindicated or otherwise unsuitable, including those who have failed to respond or have
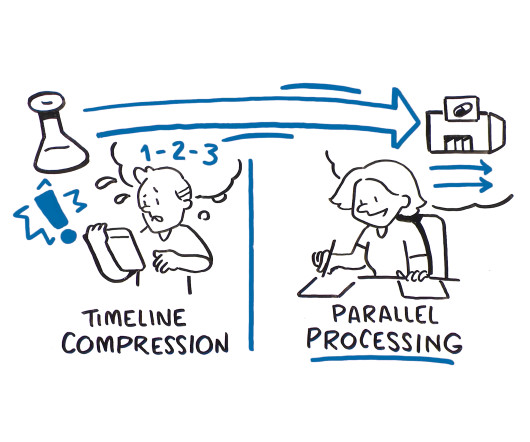
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content