Brain drug developer Alto Neuroscience prices $129M IPO
Bio Pharma Dive
FEBRUARY 2, 2024
The California biotech is the third this year to go public, following stock offerings from CG Oncology and Arrivent Biopharma.

Bio Pharma Dive
FEBRUARY 2, 2024
The California biotech is the third this year to go public, following stock offerings from CG Oncology and Arrivent Biopharma.

Pharmaceutical Technology
FEBRUARY 2, 2024
The US FDA has granted rare paediatric disease (RPD) designation for Tyra Biosciences’ TYRA-300 for treating achondroplasia.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
FEBRUARY 2, 2024
A regulatory filing shows the firm is putting together a new multibillion-dollar fund, roughly two years after raising a similar amount.

Pharmaceutical Technology
FEBRUARY 2, 2024
Graviton Bioscience has secured a strategic investment from Sanofi to propel the development of ROCK2 inhibitors for serious diseases.

Antidote
FEBRUARY 2, 2024
Endometrial cancer, also known as endometrial carcinoma or uterine cancer , is a condition that approximately 66,000 individuals are diagnosed with annually, and instances rise roughly 2% in the United States each year. Because it is often caught early, a person’s prognosis is typically good — but it is still a disheartening diagnosis that significantly impacts the lives of those who experience it.

Pharmaceutical Technology
FEBRUARY 2, 2024
With TGR-63 advancing towards the clinical stage, IGC Pharma is tackling different small molecule alternatives to treat Alzheimer’s.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
FEBRUARY 2, 2024
Carcinotech has gained funding to enter the North American market with the company's pioneering 3D-printed tumours for cancer treatments.
Pharmaceutical Commerce
FEBRUARY 2, 2024
A dive into this market reveals that the latest numbers yield promising results—a global value of a worldwide $1.6 trillion—but it will continue to be attributed to past, present, and future healthcare spending.
Pharmaceutical Technology
FEBRUARY 2, 2024
The US Food and Drug Administration (FDA) has granted fast track designation (FTD) to Biosyngen's T cell therapy BST02 for liver cancer.
Pharma Times
FEBRUARY 2, 2024
Dopamine neuron senescence is a hallmark of Parkinson’s disease

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
FEBRUARY 2, 2024
LimmaTech announced the closing of a second round of its Series A financing, securing $3m from Tenmile for vaccine technology development.
Drug Discovery World
FEBRUARY 2, 2024
Head of clinical trials at the University College London (UCL) Dementia Research Centre, Dr Cath Mummery, has been announced as the Director of a new network of dementia trial sites across the country. The UK government has authorised £49.9 ($63.4) million of new funding to improve access to clinical trials and help accelerate the development of new treatments.

Pharmaceutical Technology
FEBRUARY 2, 2024
Bristol Myers Squibb has reported a 2% revenue decrease from 2022 as it focuses on business development to counteract patent losses.

Fierce Pharma
FEBRUARY 2, 2024
As AbbVie slogs through sharp revenue declines for Humira, the company is looking to the immunology light at the end of the tunnel. | Sales of Humira—which once sat pretty as the world’s best-selling drug—plunged nearly 41% to $3.3 billion in the third quarter.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
FEBRUARY 2, 2024
England evaluates vaccine procurement and pandemic preparedness, whilst Moderna’s mRNA vaccine creeps closer to regulatory approval.

Fierce Pharma
FEBRUARY 2, 2024
In his first earnings call as Bristol Myers Squibb’s CEO, Chris Boerner laid out his plan to quickly navigate the company through a period filled with patent cliffs and new government-man | In his first earnings call as Bristol Myers Squibb’s CEO, Chris Boerner laid out his plan to quickly navigate the company through a transition period filled with patent cliffs and new government-mandated pricing pressure.

Pharmaceutical Technology
FEBRUARY 2, 2024
Common mistakes in decentralised clinical trials (DCTs) are undermining industry confidence in the model. We explore these issues.
pharmaphorum
FEBRUARY 2, 2024
Learn more about Katherine Stueland, the CEO of GeneDx, as she discusses her journey, the future of diagnostics, whilst at JP Morgan 2024.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
FEBRUARY 2, 2024
AbbVie’s Tepkinly has been recommended for the treatment of relapsed or refractory diffuse large B-cell lymphoma.

pharmaphorum
FEBRUARY 2, 2024
Our round-up of recent biotech financings is headed by a $250 million public offering for late clinical-stage biotech Vera Therapeutics, with an initial public offering (IPO) filed by CAR-T specialist Kyverna and private rounds for Eyconis, COUR Pharmaceuticals, Basking Biosciences, Enterprise Therapeutics, Halia Therapeutics and NeoPhore.

Pharma Times
FEBRUARY 2, 2024
Individuals with Jewish ancestry are six times more likely to carry a genetic fault

pharmaphorum
FEBRUARY 2, 2024
Indian pharma Hikma has agreed a $150 million settlement to resolve lawsuits claiming that it helped fuel to the opioid crisis in the US

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Fierce Pharma
FEBRUARY 2, 2024
One day after Roche said it was starting to establish Vabysmo as th | One day after Roche said it was starting to establish Vabysmo as the new standard of care in age-related macular degeneration (AMD) and diabetic macular edema (DME), Regeneron clapped back with the same claim about its new treatment—high-dose Eylea.
pharmaphorum
FEBRUARY 2, 2024
The PM Society are delighted to be running their fifth Careers Conference that will be taking place on the 21st of February 2024 between 1pm to 6pm at the Royal Society of Medicine. Please share with your colleagues and network, as this is a great opportunity for students to attend and learn more about our industry.

Pharmaceutical Commerce
FEBRUARY 2, 2024
Leading experts in the sector explore the value of third-party logistics providers when it comes to fortifying pharmaceutical supply chains.

Drug Channels
FEBRUARY 2, 2024
Today’s guest post comes from Divya Iyer, SVP of Go-to-Market Strategy at GoodRx. Divya shares highlights from GoodRx’s new research report: The GoodRx Effect: How GoodRx Improves the Economics of Healthcare. She highlights how GoodRx’s integrated pharma copay cards help to improve medication access, therapy adherence, and overall health outcomes. Click here to download the full report and learn more about partnering with GoodRx to drive patient access for your brand.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
FEBRUARY 2, 2024
The FDA is due to decide by 4th August whether to approve Adaptimmune’s afami-cel engineered T-cell therapy for advanced synovial sarcoma.

Drug Patent Watch
FEBRUARY 2, 2024
Annual Drug Patent Expirations for UBRELVY Ubrelvy is a drug marketed by Abbvie and is included in one NDA. It is available from one supplier. There are six patents protecting… The post New patent for Abbvie drug UBRELVY appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
FEBRUARY 2, 2024
The World Health Organization (WHO) has said that the burden of cancer on societies around the world is growing at an alarming rate, with a 77% increase in new cases expected by 2050, along with significant gaps in care.

Drug Patent Watch
FEBRUARY 2, 2024
Annual Drug Patent Expirations for ANNOVERA Annovera is a drug marketed by Mayne Pharma and is included in one NDA. It is available from one supplier. There are seven patents… The post New patent for Mayne Pharma drug ANNOVERA appeared first on DrugPatentWatch - Make Better Decisions.
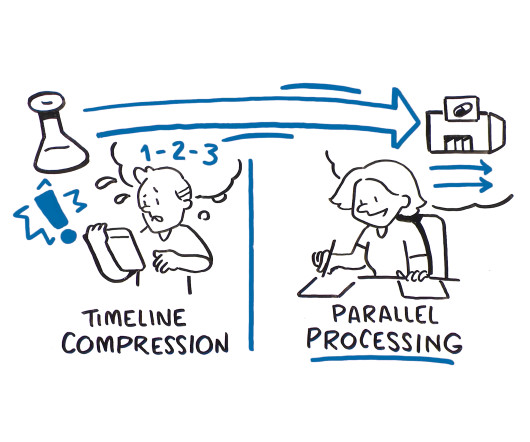
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content