European regulators probe suicide risk to Ozempic, other Novo drug Saxenda
Bio Pharma Dive
JULY 10, 2023
Three reports from Iceland spurred the EMA to evaluate whether certain Novo Nordisk drugs, including Ozempic, might cause thoughts of self harm.
Bio Pharma Dive
JULY 10, 2023
Three reports from Iceland spurred the EMA to evaluate whether certain Novo Nordisk drugs, including Ozempic, might cause thoughts of self harm.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 10, 2023
As most new parents can attest, sleepless nights don’t exactly make for the most cheerful mornings. This is why it makes little sense that total sleep deprivation temporarily resets the mood of nearly half of people with a major depressive disorder.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JULY 10, 2023
The licensing agreement for the biotech’s viral vector platform follows recent Astellas agreements with Taysha Gene Therapies and Kate Therapeutics.

Rethinking Clinical Trials
JULY 10, 2023
In an interview at the NIH Pragmatic Trials Collaboratory Steering Committee Meeting in May, Keith Marsolo, PhD, Co-Chair of the Electronic Health Records Core , reflected on data sharing experiences from the program and factors affecting the meaningful re-use of data. Keith Marsolo, PhD “The data sharing experiences from the NIH Pragmatic Trials Collaboratory Demonstration Projects have been fairly limited.

Bio Pharma Dive
JULY 10, 2023
Overcome issues with AAV vector scalability and reproducibility. Enable a wider application of gene therapies in a cost-effective and sustainable way.
Pharmaceutical Technology
JULY 10, 2023
ZyVersa Therapeutics has received a patent from the European Patent Office for VAR 200 to treat diabetic nephropathy/diabetic kidney disease.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
JULY 10, 2023
IGC Pharma has obtained a revolving line of credit worth $12m from the O-Bank’s Hong Kong branch to bolster research in Alzheimer’s disease.
Bio Pharma Dive
JULY 10, 2023
The patent is an important line of defense protecting Entresto, Novartis’ current top-selling medicine, from generic competition in the U.S.
Pharmaceutical Technology
JULY 10, 2023
The US FDA has awarded rare paediatric disease designation to NS Pharma’s NS-089/NCNP-02 to treat Duchenne muscular dystrophy.

Bio Pharma Dive
JULY 10, 2023
The pharma company will pay $30 million upfront to gain rights to an experimental therapy meant to enhance the anti-tumor effect of radiation.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
JULY 10, 2023
The US FDA has granted orphan drug designation (ODD) for Ichnos Sciences’ ISB 2001 to treat patients with multiple myeloma.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 10, 2023
Women seeking to start or expand a family can now access the Proov Confirm PdG home collection kit for assessing fertility through questhealth.com.
Pharmaceutical Technology
JULY 10, 2023
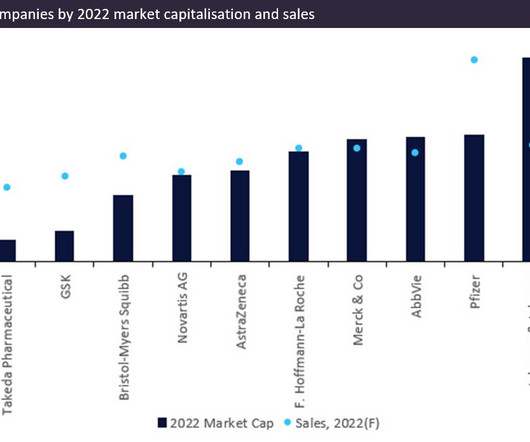
US pharma companies take the lead in the rankings of the top five companies based on market capitalisation and sales,

Fierce Pharma
JULY 10, 2023
Two cases of suicidal thoughts and one case of self-harm from users in Iceland of Novo Nordisk’s diabetes and obesity drugs have prompted the European Medicines Agency (EMA) to review the blockbust | Two cases of suicidal thoughts and one case of self-harm from users in Iceland of Novo Nordisk’s diabetes and obesity drugs have prompted the European Medicines Agency (EMA) to review the blockbuster products.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
JULY 10, 2023
Despite a loss at the district court level, Novartis is projecting a positive outlook for 2023 sales for Entresto.

XTalks
JULY 10, 2023
Euronext Brussels (UCB), a biopharma company headquartered in Brussels, Belgium, recently announced that the US Food and Drug Administration (FDA) has granted approval for its drug, Rystiggo (rozanolixizumab), for the treatment of generalized myasthenia gravis (gMG). UCB specializes in the discovery and development of innovative medicines for severe diseases that affect the immune system or central nervous system.
Pharmaceutical Technology
JULY 10, 2023
Despite significant need for novel therapeutics to treat triple negative breast cancer, this is proving difficult.

XTalks
JULY 10, 2023
In a twist of fate, Monster Energy may soon become both the savior and executioner of its competitor, Bang Energy. As reported by the Wall Street Journal , Monster Energy is poised to acquire its smaller rival for $362 million through a bankruptcy court agreement. This transaction is pending approval from the bankruptcy court and the Federal Trade Commission (FTC), which is currently reviewing the potential sale; however, if given the green light, this deal could potentially be beneficial for bo

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Pharmaceutical Technology
JULY 10, 2023
The belated decision follows RSV vaccine approvals in America and the European Union from earlier this year.

Outsourcing Pharma
JULY 10, 2023
Updated guidelines for risk-based quality management (RBQM) are much more than just for monitoring purposes and can show quality by design concepts, save immeasurable time on manpower and shows there has been a complete mindset shift within the last 15 years, CluePoints says.

Pharmaceutical Technology
JULY 10, 2023
Janssen will have a worldwide licence to develop and commercialise Nanobiotix’s oncology drug, NBTXR3.

Fierce Pharma
JULY 10, 2023
Just days after sewing up a pair of $897 million deals with Pfizer, Samsung Biologics has added a few hundred million dollars more to its partnership cash pile—this time courtesy of an expanded pac | Samsung Biologics said in a Monday regulatory filing that it’s inked a $390.9 million deal to help crank out Novartis drugs. The latest production pact builds on an earlier tie-up worth $81 million.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Technology
JULY 10, 2023
Leqvio has received approval for adjunct treatment in patients with elevated LDL cholesterol.

Pharma Times
JULY 10, 2023
Period to remission was shortened with esketamine nasal spray in both sub-groups within study - News - PharmaTimes
XTalks
JULY 10, 2023
With the remarkable success of its semaglutide products Wegovy and Ozempic for weight loss and type 2 diabetes, respectively (the latter also for weight loss through off-label prescribing), Novo Nordisk is cracking down on pharmacies making compounded versions of the glucagon-like peptide-1 (GLP-1) receptor agonist drugs. In light of dupes making their way across pharmacies, the Danish company has launched a new round of lawsuits in the US against those that are making illegal versions of their

Pharma Times
JULY 10, 2023
Therapy is the first respiratory syncytial virus vaccine specifically developed for older adults - News - PharmaTimes

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
BioPharma Reporter
JULY 10, 2023
Pfizer has made a $25 million equity investment in leading clinical-stage biopharmaceutical company, Caribou Biosciences.

BioSpace
JULY 10, 2023
After its Biologics License Application was rejected by the FDA, BrainStorm’s Phase III data suggest its amyotrophic lateral sclerosis candidate significantly lowers neurofilament light chain levels.

Pharma Marketing Network
JULY 10, 2023
In today’s digital landscape, a website serves as a vital touchpoint for businesses to connect with their target audience. However, over time, a website can become outdated, lose relevance, or fail to resonate with visitors. When done strategically, rebranding a website can breathe new life into your online presence and significantly boost traffic.

BioSpace
JULY 10, 2023
The biotech will supply Canada with fewer doses of its coronavirus vaccine while reaching a financial agreement for the forfeiting of certain doses previously scheduled for delivery.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content