Moderna, Merck advance cancer vaccine into late-stage test
Bio Pharma Dive
JULY 26, 2023
The companies are enrolling people with melanoma in a Phase 3 trial aimed at testing whether the shot can prevent disease recurrence.
Bio Pharma Dive
JULY 26, 2023
The companies are enrolling people with melanoma in a Phase 3 trial aimed at testing whether the shot can prevent disease recurrence.

Pharmaceutical Technology
JULY 26, 2023
Eli Lilly has extended the tender offer expiration date for the acquisition of all Dice Therapeutics in a deal valued at $2.4bn.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
JULY 26, 2023
Professor Carl Bergstrom In this Friday’s PCT Grand Rounds, Professor Carl Bergstrom of the University of Washington will present “How Can Researchers Fight Misinformation About Medicine?” The Grand Rounds session will be held on Friday, July 28, 2023, at 1:00 pm eastern. Bergstrom is a professor of biology at the University of Washington.

Bio Pharma Dive
JULY 26, 2023
In challenging AbbVie for share of a $19 billion drug market, competitors are testing whether high upfront discounts or behind-the-scenes rebates can win them an advantage.

Pharmaceutical Technology
JULY 26, 2023
In the global pharmaceutical industry, there were 92 private equity deals announced in Q2 2023, worth a total value of $17.3bn, according to GlobalData's Deals Database.

Bio Pharma Dive
JULY 26, 2023
The cost cuts at the nearly 30-year-old biotech are part of plans to seek a “strategic transaction” for the company’s only remaining drug prospect.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
JULY 26, 2023
Led by Vivek Ramaswamy's brother, Kriya has now raised over $600 million to fund gene therapy research spanning ophthalmology, neurology and metabolic disease.
Pharmaceutical Technology
JULY 26, 2023
The US Food and Drug Administration (FDA) has approved Tarsus Pharmaceuticals’ Xdemvy for the treatment of Demodex blepharitis.
Bio Pharma Dive
JULY 26, 2023
The startup is the latest young company to emerge with plans to make production of cell-based medicines easier for researchers and biotechs.
Pharmaceutical Technology
JULY 26, 2023
Bioengineering company Ossium Health has secured $52m in Series C funding for expanding its bone marrow stem cell bank.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
JULY 26, 2023
The startup, which initially aims to make cancer medicines, claims to have a new twist on covalent drug research.
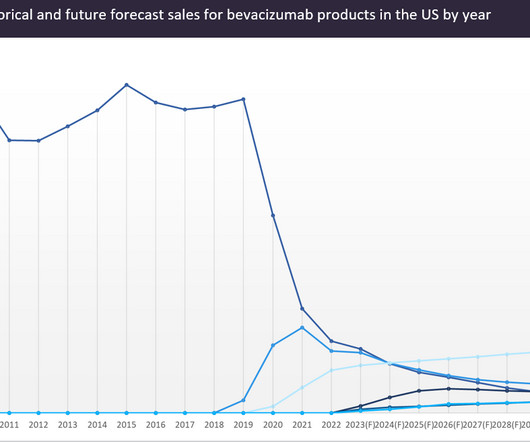
Pharmaceutical Technology
JULY 26, 2023
Roche’s anti-VEGF monoclonal antibody Avastin (bevacizumab) is one of the most successful oncology drugs of all time.

ACRP blog
JULY 26, 2023
As leaders in the clinical research enterprise seek to expand the public’s understanding of clinical trials and their potential benefits for healthcare at the personal, community, and national levels, communications tactics cannot remain stuck at the one-on-one rate of physician referrals or patient recruiters reaching out to individual potential volunteers for real progress to be seen.

Pharmaceutical Technology
JULY 26, 2023
Intrommune Therapeutics has completed the last patient last visit for its desensitisation candidate INT301.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

ProRelix Research
JULY 26, 2023
Ensuring the safety and efficacy of new drugs, medical devices, and biological products does not end once the treatment is approved or is on the market but extends to the […] The post Pharmacovigilance (PV) Services at a Glance appeared first on ProRelix Research.

XTalks
JULY 26, 2023
The US Food and Drug Administration (FDA) has granted approval to Daiichi Sankyo’s Vanflyta (quizartinib) for newly diagnosed patients with FMS-like tyrosine kinase 3 receptor-internal tandem duplication ( FLT3 -ITD) positive acute myeloid leukemia (AML). FLT3 -ITD mutations occur in about a quarter of all AML cases. Vanflyta is the first and only FLT3 inhibitor approved in the US for FLT3 -ITD positive AML and across all three treatment phases: induction, consolidation and maintenance.

Fierce Pharma
JULY 26, 2023
As with Novo Nordisk’s popular offering for obesity, Wegovy, demand for Eli Lilly’s dual GIP/GLP-1 agonist Mounjaro appears to be outpacing supply once again. | The U.S. Food and Drug Administration has added a fourth dose of Lilly’s popular new diabetes med to its drug shortage database. The agency also expects supply issues with three other doses to persist longer than previously thought.

Pharma Marketing Network
JULY 26, 2023
The Food and Drug Administration (FDA) plays a critical role in regulating the pharmaceutical industry and ensuring that medications and medical devices marketed to the public are safe, effective, and appropriately labeled. One of the FDA’s enforcement mechanisms is the issuance of marketing violation letters to pharmaceutical companies found in violation of advertising and promotion regulations.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

BioPharma Reporter
JULY 26, 2023
Leslie Orne joined Trinity Life Sciences in 2001 and was recently appointed CEO and president of the fast-growing healthcare consultancy. She previously held the title of chief commercial officer (CCO) and president.
Fierce Pharma
JULY 26, 2023
With a European approval for chronic kidney disease nod in hand, Boeringer Ingelheim and Eli Lilly's Jardiance is looking to catch up to AstraZeneca's Farxiga in this use and continue growing its r | After recent label expansions in the U.S. and Europe paid off with a sizable sales increase for Jardiance, the drug has added a third use to its European label.

Pharma Times
JULY 26, 2023
The new company’s core mission is to boost patient access to disease-modifying treatments - News - PharmaTimes

Fierce Pharma
JULY 26, 2023
Despite a positive opinion from local drug reviewers, GSK has decided not to bring its FDA-approved oral anemia drug daprodustat to Europe—or any additional countries for that matter. | Despite a positive opinion from local drug reviewers, GSK has decided not to bring its FDA-approved oral anemia drug daprodustat to Europe—or any additional countries for that matter.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Pharma Times
JULY 26, 2023
The advice includes a national rollout of the HCV GP champion programme and further testing - News - PharmaTimes
BioSpace
JULY 26, 2023
In a recent study of the U.S. states with the best and worst job markets, nine came out on top. Read on for details about the life sciences industry and jobs available in each state.

Fierce Pharma
JULY 26, 2023
Alkermes brought in $617 million during the second quarter, including $248 million of back royalties on Johnson & Johnson's Invega franchise. The company recently won an arbitration case against the pharma giant.

BioSpace
JULY 26, 2023
Amid the impending drug pricing pressures from the Inflation Reduction Act, Roche is discontinuing a mid-stage hemophilia A gene therapy candidate and four early-stage hopefuls.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
JULY 26, 2023
More than 2.4 million people in the United States use warfarin to keep their blood from clotting after a heart attack, stroke or other serious thromboembolic complication. | The FDA signed off on Octapharma's blood coagulant Balfaxar as a treatment to counter the effects of commonly used blood thinner warfarin.

BioSpace
JULY 26, 2023
With partner Biogen mum on zuranolone’s prospects as an FDA August 5 review deadline approaches, Sage Therapeutics’ stock fell Wednesday to its lowest level in months.
Pharmaceutical Commerce
JULY 26, 2023
Updated deal will cover exclusive US commercialization for several of Alvotech’s biosimilars.

Fierce Pharma
JULY 26, 2023
Expectations are sky high for GSK’s entry this fall into the new respiratory syncytial virus (RSV) vaccine market. | In a quarter fueled by sales of Shingrix and its HIV medicines, GSK topped revenue expectations and bumped up its sales projection for the year. While the company anxiously awaits the launch this fall of RSV vaccine Arexvy, it is tamping down expectations of an immediate boom in sales for the potential blockbuster.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content