Simtra BioPharma to invest more than $250m in US facility
Pharmaceutical Technology
MARCH 1, 2024
Simtra BioPharma has announced plans invest $250m for an expansion of its sterile fill/finish manufacturing site in Bloomington, Indiana, US.

Pharmaceutical Technology
MARCH 1, 2024
Simtra BioPharma has announced plans invest $250m for an expansion of its sterile fill/finish manufacturing site in Bloomington, Indiana, US.

Bio Pharma Dive
MARCH 1, 2024
The expert committee is discussing whether, for certain older adults, to make a universal recommendation for vaccination, rather than the current policy of “shared decisionmaking.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MARCH 1, 2024
Experts say that dry granulation is 10x more efficient than wet granulation, which could make substantial progress towards making this pharmaceutical process greener.

Bio Pharma Dive
MARCH 1, 2024
The restructuring comes ahead of a key study readout for Gritstone, which, like Moderna, is developing a type of personalized vaccine for cancer.
Pharmaceutical Technology
MARCH 1, 2024
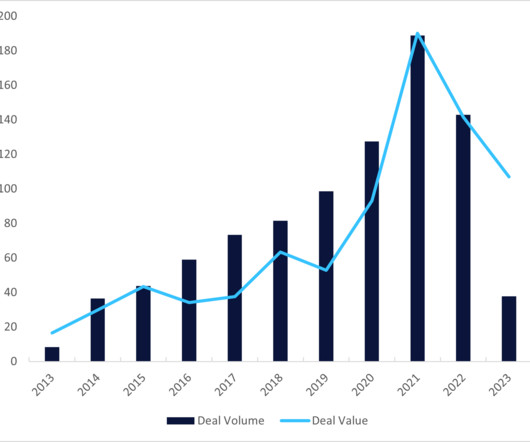
During 2023, funding decreased by 43.2% compared to 2022 and by 52.3% compared to 2021, attributed to macroeconomic pressures.

Bio Pharma Dive
MARCH 1, 2024
Reeling from a difficult year financially, the pharma unveiled a new oncology division it says will produce eight billion-dollar medicines by 2030.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
MARCH 1, 2024
The cash will be used to advance development of FOG-001, the company’s lead drug that’s now being tested in an early-stage trial for solid tumors.

Pharmaceutical Technology
MARCH 1, 2024
1910 Genetics has entered an agreement with Microsoft, aiming to revitalise pharmaceutical research and development (R&D) productivity.

Fierce Pharma
MARCH 1, 2024
Moderna is laying off employees within its manufacturing unit, with the move tied to a resizing of its COVID production work. | Moderna is laying off some employees within its manufacturing unit after shaving COVID production costs. The company previously disclosed plans to rightsize its COVID vaccine production footprint.

Pharmaceutical Technology
MARCH 1, 2024
US FDA placed a partial clinical hold on HIV programme and a full hold on Covid-19 trials for CytoDyn’s monoclonal antibody, leronlimab.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Fierce Pharma
MARCH 1, 2024
It’s time to add another vaccine to the shortage list in the U.S. | It’s time to add another vaccine to the shortage list in the United States as the CDC has urged healthcare providers to conserve their supply of tetanus shots. The warning from the national public health agency comes after one of the two providers of tetanus shots in the U.S., MassBiologics, discontinued production of its tetanus and diphtheria vaccine.
Pharmaceutical Technology
MARCH 1, 2024
In addition to r/r multiple myeloma, the biotech is evaluating the drug as a treatment for advanced solid tumours alongside Keytruda.
pharmaphorum
MARCH 1, 2024
There is a pressing need for companies to transition their EU clinical trials that are ongoing under the “old” regulatory framework of the Clinical Trials Directive to the Clinical Trials Regulation. The risk for those that don’t meet the 30th January 2025 deadline for transition is that they will lose their legal basis.

Pharmaceutical Technology
MARCH 1, 2024
The UK MHRA has granted marketing authorisation for a new formulation of Amgen’s XGEVA to prevent serious bone-related complications.

pharmaphorum
MARCH 1, 2024
Pfizer says new oncology division will generate eight new blockbusters by 2030, in first R&D update after its $43bn takeover of Seagen.

Pharmaceutical Technology
MARCH 1, 2024
BlossomHill has secured $100m in a Series B financing round to advance its programmes targeting cancer and autoimmune diseases.

Fierce Pharma
MARCH 1, 2024
Less than three weeks after a Texas judge tossed a lawsuit b | Less than three weeks after a Texas judge tossed a lawsuit by industry lobbying group PhRMA that challenged the constitutionality of the Inflation Reduction Act, a federal court in Delaware has done the same with a similar action brought by AstraZeneca.
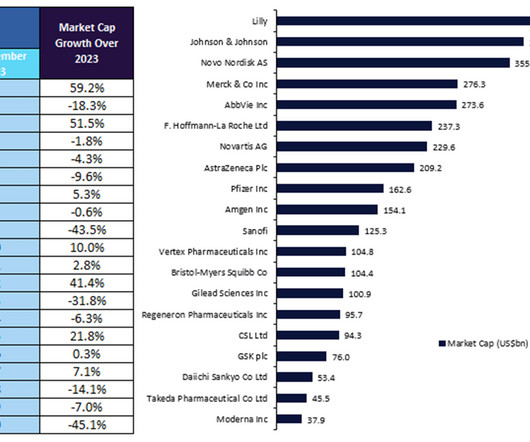
Pharmaceutical Technology
MARCH 1, 2024
The top 20 biopharmaceutical companies saw prominent shifts in market capitalisation over 2023 despite various obstacles.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

pharmaphorum
MARCH 1, 2024
Stay up to date with the latest news in the life sciences industry with highlights from clinical trials involving CSL, Arcturus, Sanofi, Nexviazyme, and REGENXBIO from the week of 5th to 9th February 2024.

Intouch Solutions
MARCH 1, 2024
To honor Black History Month, we’re proud to share some of the inspiring and impactful events that were hosted throughout the month. This year’s theme was “Mapping the Black Experience,” where we took a deeper look into exploring Black Culture and its history through art, food and travel. Our Black Professional Network co-chairs, Eric Terry, Director, Quality Control and Martika Chappell, People Specialist, focused this year’s theme on what it means to move through the world while Black, aiming

pharmaphorum
MARCH 1, 2024
Rare Disease Day saw the debut of a film that aims to raise awareness of cerebral adrenoleukodystrophy (cALD), a devastating and fatal condition with no pharmacological treatments available.
Pharma Times
MARCH 1, 2024
Different factors, such as illness duration and virus variant, impacted patients cognitive abilities

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

pharmaphorum
MARCH 1, 2024
A new formulation of Amgen’s cancer treatment Xgeva is the first to be approved in the UK under a new procedure designed to speed up access to new therapies.

Fierce Pharma
MARCH 1, 2024
The FDA recently asked the six marketed CAR-T therapies to add a new boxed warning item on their labels to reflect the risk of secondary T-cell cancers. | The FDA recently asked the six marketed CAR-T therapies to add a new boxed warning item on their labels to reflect the risk of secondary T-cell cancers. This week on "The Top Line," Fierce Pharma’s Angus Liu talks with regulatory experts to understand the rationale behind the FDA’s decision and to discuss its implications for CAR-T c

Pharma Times
MARCH 1, 2024
TMR-CT will help doctors select treatment and predict the spread of lung cancer in patients

Fierce Pharma
MARCH 1, 2024
In the latest shake-up amid waves of changes at Bayer, the company is planning to add new expertise to its board room, including a prominent activist investor. | The company's supervisory board proposed the appointments of activist investor Jeffrey Ubben, legal expert Lori Schecter and biotech leader Nancy Simonian, M.D. Bayer also swapped in a new head of its consumer health division.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Outsourcing Pharma
MARCH 1, 2024
Cresset, a leading provider of integrated in silico solutions for drug discovery, announced last month (February 21) a global collaboration with Enamine, one of the front providers of chemical building blocks and drug discovery services.

Fierce Pharma
MARCH 1, 2024
With a new FDA nod, BeiGene has filled the follicular lymphoma approval gap for BTK inhibitors. | BeiGene has filled the follicular lymphoma gap for BTK inhibitors with an FDA approval for Brukinsa about a year after AbbVie and Johnson & Johnson's Imbruvica stumbled in a phase 3 trial. But tough competition with CAR-T therapies and bispecifics lies ahead.

pharmaphorum
MARCH 1, 2024
German biotech 4SC has filed its oral HDAC inhibitor Kinselby as a maintenance therapy for rare cancer CTCL in the EU

Outsourcing Pharma
MARCH 1, 2024
Metabolon, Inc., a prominent figure in metabolomics solutions, has launched a seminal integrated bioinformatics platform, aiming to redefine metabolomics analysis in life sciences research.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content