Engineering team develops multifunctional tendon-mimetic hydrogels
Medical Xpress
APRIL 22, 2023
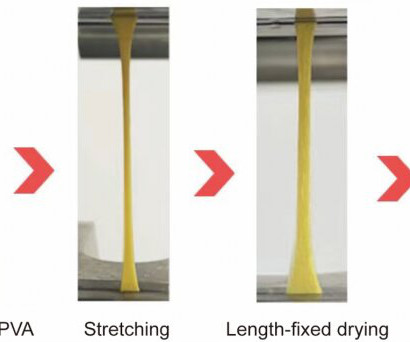
Repairing or replacing injured tendons or similar load-bearing tissues represents one of the major challenges in clinical medicine. Natural tendons are water-rich tissues exhibiting outstanding mechanical strength and durability. Their mechanical properties originate from sophisticated microscale structures involving stiff collagen fibrils aligned in parallel and interlaced with soft water-retaining biopolymers.




























Let's personalize your content