Roche shuffles early stage pipeline as it joins obesity rush
Bio Pharma Dive
FEBRUARY 1, 2024
Roche said it was shelving those drugs in favor of experimental programs to treat obesity that it gained from its takeover of Carmot Therapeutics.

Bio Pharma Dive
FEBRUARY 1, 2024
Roche said it was shelving those drugs in favor of experimental programs to treat obesity that it gained from its takeover of Carmot Therapeutics.

Pharmaceutical Technology
FEBRUARY 1, 2024
Merck & Co (MSD) has reported a 1% revenue increase from 2022, and may face setbacks in the next few years from top-selling drugs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
FEBRUARY 1, 2024
François-Xavier Roger will replace current CFO Jean-Baptiste Chasseloup de Chatillon as Sanofi continues a research pivot and cost-cutting drive.

Pharmaceutical Technology
FEBRUARY 1, 2024
Novartis has announced net income soaring by 62% to $8.6bn for the full year 2023 (FY 2023) from the previous year’s $6.04bn.

Bio Pharma Dive
FEBRUARY 1, 2024
The agreement gives Protagonist an experienced hand in Takeda, which can help the former commercialize its product.
Pharmaceutical Technology
FEBRUARY 1, 2024
The French government grant will cover preclinical and clinical research for Vivet’s gene therapy for cerebrotendinous xanthomatosis.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
FEBRUARY 1, 2024
The financing extension brings Series B total funding to $39.5m, helping advance the UK company’s lead DNA MMR protein candidate.
Fierce Pharma
FEBRUARY 1, 2024
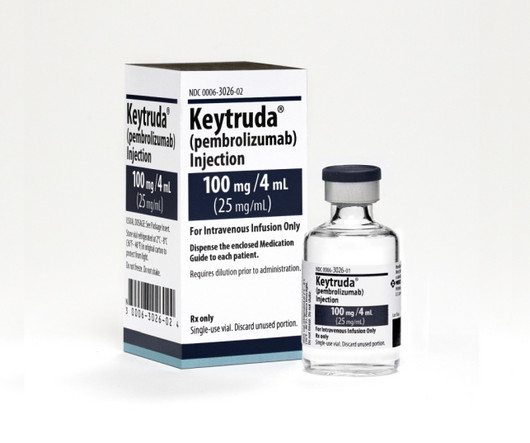
With Merck reporting a whopping $25 billion sales haul for | With Merck reporting a whopping $25 billion sales haul for Keytruda on Thursday, the PD-1 cancer superstar appears to be the world’s top-selling drug in 2023. Keytruda looks set to take over the top spot from Pfizer and BioNTech’s Comirnaty, which ruled the previous two years, scoring sales of $55.9 billion in 2022 and $55.1 billion in 2021.

Pharmaceutical Technology
FEBRUARY 1, 2024
Almirall has entered a strategic collaboration with Microsoft to steer innovation and digital transformation in dermatology.

pharmaphorum
FEBRUARY 1, 2024
Explore the current state of pharmaceutical meetings and events in 2024, including the shift from in-person to virtual and hybrid formats. Discover how the industry is embracing diversity, equity, and inclusion in these gatherings.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
FEBRUARY 1, 2024
The UK MHRA has approved the use of GSK’s momelotinib (Omjjara) for moderate to severe anaemia in adult patients with myelofibrosis.

pharmaphorum
FEBRUARY 1, 2024
A phase 2 trial of Lundbeck’s alpha-synuclein-targeting drug Lu AF82422 has failed to reach statistical significance in patients with multiple system atrophy (MSA), although the company says there are encouraging “signals of efficacy” in the data.
Pharmaceutical Technology
FEBRUARY 1, 2024
Takeda has entered a global licence and partnership agreement with Protagonist Therapeutics for developing and marketing rusfertide.

pharmaphorum
FEBRUARY 1, 2024
On today's podcast, brought to you by Kadans Science Partners, host Jonah Comstock is joined by Bradley Hardiman, Astellas Pharma’s Senior Director of Business Development; Mike Murray, head of Murray International Partners Ltd; and Mairi Dillon, Kadans' Ecosystem Manager for UK & Ireland.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
FEBRUARY 1, 2024
Getting new drugs out of the research phase, and into clinical studies and commercialization, is an odyssey in quality control
BioPharma Reporter
FEBRUARY 1, 2024
Upon hearing the phrase âcancer treatmentâ, the first word that can typically spring to mind is chemotherapy â a primary therapy which uses powerful chemicals to destroy rapidly growing cancer cells in the body.

Pharmaceutical Technology
FEBRUARY 1, 2024
GlobalData reports that HCPs predict a continued increase in drug prices, as inflationary pressures rise.

Fierce Pharma
FEBRUARY 1, 2024
Even after the acquisitions of Prometheus and Acceleron, Merck is ready to make more deals in the $1 to $15 billion range, according to CEO Rob Davis.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
FEBRUARY 1, 2024
Our Awards and Rankings report is available now to download
Outsourcing Pharma
FEBRUARY 1, 2024
A company building a pipeline of small molecule drugs targeting novel proteins across the DNA mismatch repair (pathway) has announced a further extension to its series B financing round.

Pharmaceutical Technology
FEBRUARY 1, 2024
Our Awards and Rankings report is available now to download

Outsourcing Pharma
FEBRUARY 1, 2024
A husband-and-wife team keen to find a solution to an âNHS in crisisâ have secured $1.5 million in seed funding for their UK-based startup providing AI-powered clinical and drug prescription services for minor conditions.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

pharmaphorum
FEBRUARY 1, 2024
As cyberattacks continue to target the healthcare sector, it is crucial to look back at cybersecurity incidents from the past year and prepare for the challenges that lie ahead. This retrospective discusses the impact on patients, health systems, insurers, and vendors, and highlights the importance of cybersecurity measures in protecting sensitive healthcare information.

Fierce Pharma
FEBRUARY 1, 2024
Despite litigation and denouncements from the biopharma industry, Medicare price negotiations under the Inflation Reduction Act are rolling on. | Despite litigation and denouncements from the biopharma industry, Medicare price negotiations under the Inflation Reduction Act are rolling on. And today, the government is sending out its first round of offers on affected drugs.

BioPharma Reporter
FEBRUARY 1, 2024
Cartherics, a biotechnology company developing immune cell therapies for the treatment of cancer, has completed a pre-investigational new drug (pre-IND) meeting with the FDA for a phase 1/2 clinical trial of its cell therapy product, CTH-401 for the treatment of ovarian cancer.
Fierce Pharma
FEBRUARY 1, 2024
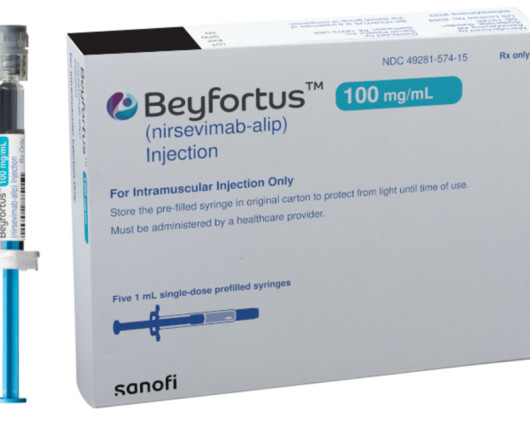
As Sanofi strives to become a global immunology leader, it's also busy making the most of a crucial respiratory launch with partner AstraZeneca. | As Sanofi strives to become a global immunology leader, it's also busy making the most of a crucial respiratory launch with partner AstraZeneca. On both fronts, Sanofi expects to see dividends in 2024.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Pharma Times
FEBRUARY 1, 2024
Tumours with high mtDNA mutations more likely to respond to Opdivo

Fierce Pharma
FEBRUARY 1, 2024
As Roche CEO Thomas Schinecker sees it, the Swiss pharma made a “major achievement” in 2023. Despite a sharp decline in COVID-related sales, the company was able to grow overall revenues | As Roche CEO Thomas Schinecker sees it, the Swiss pharma made a “major achievement” in 2023. Despite a sharp decline in COVID-related sales, the company was able to grow overall revenues thanks in large part to the performance of eye disease drug Vabysmo.

pharmaphorum
FEBRUARY 1, 2024
Almirall has formed a three-year alliance with Microsoft designed to accelerate the discovery and development of personalised dermatology therapies.

Fierce Pharma
FEBRUARY 1, 2024
After GSK spent the latter half of 2023 warding off concerns about the swelling Zantac product liability litigation, the company is back at the settlement table in 2024. | After GSK spent the latter half of 2023 warding off concerns about the swelling Zantac product liability litigation, the company is back at the settlement table in 2024.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content