Atara’s cell therapy approach to MS falls short in study
Bio Pharma Dive
NOVEMBER 9, 2023
Shares in the biotech tumbled by 75% as results from a Phase 2 trial showed more improvement in participants given a placebo.
Bio Pharma Dive
NOVEMBER 9, 2023
Shares in the biotech tumbled by 75% as results from a Phase 2 trial showed more improvement in participants given a placebo.

Pharmaceutical Technology
NOVEMBER 9, 2023
GlobalData analyst Jemima Walker discusses key topics around cloud computing for the pharmaceutical industry.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 9, 2023
An ingredient once commonly used in citrus-flavored sodas to keep the tangy taste mixed thoroughly through the beverage could finally be banned for good across the US.

Pharmaceutical Technology
NOVEMBER 9, 2023
Every year, the patient recruitment landscape is getting larger and more multi-faceted. The good news is that sponsors now have a wide range of options at their fingertips; the challenge is that it’s becoming harder to oversee the flow of patients and understand what is and isn’t working – and therefore hard to make meaningful decisions that improve the trajectory of recruitment for your trial.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 9, 2023
In an effort to ensure a stable and cost-effective supply chain for active pharmaceutical ingredients (APIs) in the country, the Department of Pharmaceuticals (DoP) may look at tapping the potential of international collaborations for sourcing of raw materials and manufacturing of ingredients in the country.

Bio Pharma Dive
NOVEMBER 9, 2023
AstraZeneca is paying $185 million upfront to gain access to an experimental GLP-1 drug from China-based biotech Eccogene.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.

Pharmaceutical Technology
NOVEMBER 9, 2023
Zepbound (tirzepatide) has been approved by the FDA approval and is expected to be launched in the US by the end of 2023.

Antidote
NOVEMBER 9, 2023
Before any new treatment or therapy is able to be used by the patient population, it must go through the process of a clinical trial — this is the case for any new prescription drug, but also true for over-the-counter medications, medical devices, and more.

Pharmaceutical Technology
NOVEMBER 9, 2023
New sustainable packaging technologies aimed at children and seniors need to be prioritised to avoid drug recalls.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
BioPharma Reporter
NOVEMBER 9, 2023
The proof-of-concept trial, dubbed IRIS-RA, is the first to investigate a treatment for rheumatoid arthritis that blocks a protein called neonatal Fc receptor (FcRn). The antibody drug nipocalimab, developed by the Janssen Pharmaceutical Companies of Johnson & Johnson, has shown early promise for treating rheumatoid arthritis (RA) in a phase 2a trial.

Pharmaceutical Technology
NOVEMBER 9, 2023
Patients in the US who were previously treated for metastatic colorectal cancer are now cleared to use Takeda’s chemotherapy-free drug.

Intouch Solutions
NOVEMBER 9, 2023
Back in 2003, drugs from large pharma companies made up 36% of the late-stage R&D pipeline. By 2018, that share had fallen by about half. Meanwhile, the number of companies launching their first drug during the decade preceding 2018 more than tripled. These data are astonishing, given the perception that Big Pharma is the main driver of biopharma innovation.

Pharmaceutical Technology
NOVEMBER 9, 2023
Ascidian Therapeutics has raised $40m Series A extension funding round to progress the development of its lead and pipeline programmes.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Intouch Solutions
NOVEMBER 9, 2023
A group of EVERSANA INTOUCHers recently volunteered at the Leukemia & Lymphoma Society’s (LLS) Light The Night walk in Mercer County, NJ. The purpose of Light The Night is to gather as a community to celebrate, honor, and remember those touched by blood cancers. Friends, families, schools, corporate teams, and sponsors joined together to bring light to the darkness of cancer.
Pharmaceutical Technology
NOVEMBER 9, 2023
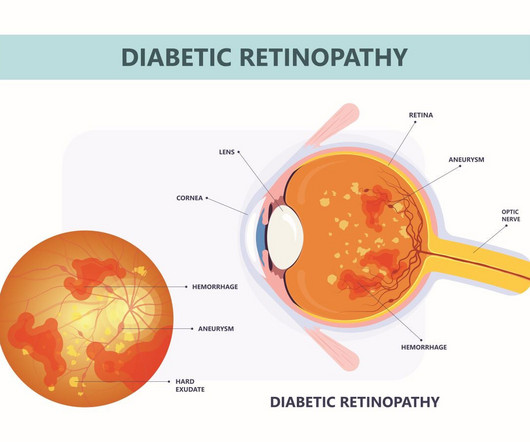
ALTITUDE provided results on the efficacy and safety of ABBV-RGX-314 to treat diabetic retinopathy over the course of 12 months.

Pharma Times
NOVEMBER 9, 2023
The WID-qEC test successfully identified 91% of womb cancer cases - News - PharmaTimes

Pharmaceutical Technology
NOVEMBER 9, 2023
Avid Bioservices has entered into a partnership with CIRM to manufacture AAV and other cell and gene therapy (CGT) programmes.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
NOVEMBER 9, 2023
Thanks to a Takeda approval Thursday, patients now have a drug to tackle a rare inherited blood clotting disorder. | The FDA has given a green light to Takeda’s Adzynma, the first recombinant protein product as a preventative or on demand enzyme replacement therapy in adults and kids with congenital thrombotic thrombocytopenic purpura.
Pharmaceutical Technology
NOVEMBER 9, 2023
By Iain Little, IRT Practice Lead at Tenthpin & Dan Silva Partner at Tenthpin With the method of managing the…

Pharma Times
NOVEMBER 9, 2023
The campaign was first launched to coincide with the 75th anniversary of the NHS - News - PharmaTimes

Pharmaceutical Technology
NOVEMBER 9, 2023
Eccogene will receive $185m upfront and up to $1.82bn in payments from AstraZeneca for the small molecule GLP-1 agonist.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Cloudbyz
NOVEMBER 9, 2023
Adverse events in clinical settings often pose a puzzle for pharmacovigilance professionals, demanding a careful examination of the complex multifactorial nature of these occurrences. The challenges in causality assessment are numerous, reflecting the intricate landscape of clinical safety pharmacovigilance. 1. Standardized Assessment Tools and Expert Review Committees: Adopting standardized causality assessment tools, such as the Naranjo algorithm or WHO causality assessment, and establishing e

Pharmaceutical Technology
NOVEMBER 9, 2023
Teva Pharmaceutical has posted net income attributable to the company of $80m for Q3 2023 as against $56m in Q3 2022.
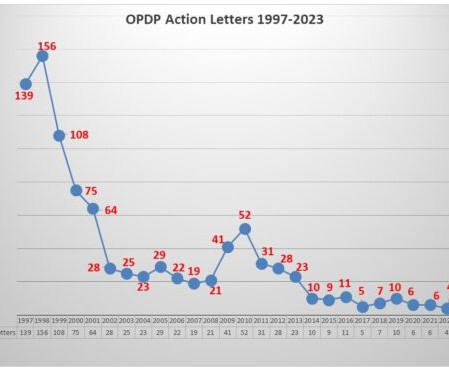
Eye on FDA
NOVEMBER 9, 2023
While enforcement has been at a low ebb for quite some time with FDA’s Office of Prescription Drug Promotion (OPDP), this week took a different turn with the posting of two new untitled letters sent October 31. That brings the total number of letters issued by OPDP to five this year – one Warning Letter that came out in August respecting a sales aid used in promoting a treatment for COPD, and 4 untitled letters – one in June, one in August, and now two in October.
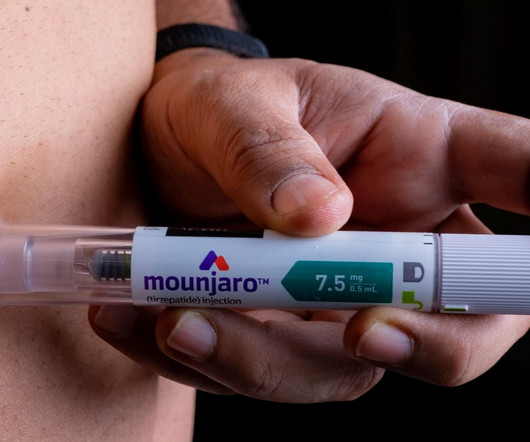
Pharmaceutical Technology
NOVEMBER 9, 2023
The UK MHRA has granted authorisation for Eli Lilly’s Mounjaro (tirzepatide) for weight loss and weight management.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Fierce Pharma
NOVEMBER 9, 2023
AstraZeneca licensed an oral GLP-1 agonist from China's Eccogene. Takeda won FDA approval for the Hutchmed-developed colorectal cancer drug Fruzaqla. | AstraZeneca licensed an oral GLP-1 agonist from China's Eccogene. Takeda won FDA approval for Hutchmed-developed colorectal cancer drug Fruzaqla. BioNTech bought a PD-L1xVEGF bispecific antibody from Chinese biotech Biotheus.

pharmaphorum
NOVEMBER 9, 2023
Medicine, Media, Metamorphosis: How M3 is championing medical communications for modern healthcare Mike.
Drug Discovery World
NOVEMBER 9, 2023
DDW Editor Reece Armstrong speaks to Christian Leisner , Chief Executive Officer of antibody therapeutics’ developer CDR-Life about methods to increase access to antigens and improving immunotherapy treatments. RA: Why are existing immunotherapies restricted to certain patient populations? CL: Immunotherapies, principally anti-PD-1/-L1 checkpoint inhibitors, have shown therapeutic benefit in improving the prognosis of patients but only in a select handful of solid cancers.
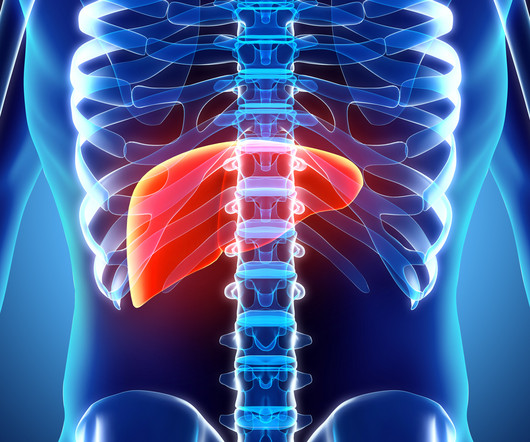
Fierce Pharma
NOVEMBER 9, 2023
AstraZeneca chases another liver cancer nod for Imfinzi with early phase 3 win, scraps Imfinzi-Lynparza combo study in lung cancer zbecker Thu, 11/09/2023 - 11:02

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content