Digital health tech: a solution to substance use disorders?
Pharmaceutical Technology
JULY 4, 2023
Substance use disorders leave people with long-term negative mental and physical health implications and can lead to death.

Pharmaceutical Technology
JULY 4, 2023
Substance use disorders leave people with long-term negative mental and physical health implications and can lead to death.

Outsourcing Pharma
JULY 4, 2023
Drugs like Annovis Bioâs buntanetap and prasinezumab have the potential to ârevolutionize the treatment of Parkinsonâs Diseaseâ says analytics company, GlobalData, an analytics company, but it is a complex road ahead.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JULY 4, 2023
While drug shortages have been a major cause for concern over the past few years, medications to treat cancer are in particularly short supply.
Pharma Tutor
JULY 4, 2023
Reality Test of Medicine Shop without Registered Pharmacist admin Tue, 07/04/2023 - 15:39 ABOUT AUTHOR Dr. R. S. Thakur Renowned Professor of Pharmaceutical Fraternity & Former Member of Pharmacy Council of India. Email : drramsthakur@gmail.
Pharmaceutical Technology
JULY 4, 2023
Abeona Therapeutics has raised $25m from its current select investors to launch and commercialise its cell therapy, EB-101.

Outsourcing Pharma
JULY 4, 2023
Clerkenwell Health, a specialist clinical research organisation (CRO) focused on supporting clients with the design and delivery of psychedelic-assisted therapy trials, is sponsoring the upcoming Psych Symposium 2023 on Thursday 6 July in London.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharma Times
JULY 4, 2023
Therapy has been developed to reduce the frequency, duration and severity of migraine attacks - News - PharmaTimes

Pharmaceutical Technology
JULY 4, 2023
The US FDA has issued a complete response letter to Amneal Pharmaceuticals, declining to approve its IPX203 to treat Parkinson’s disease.

Drug Discovery World
JULY 4, 2023
New data from Boehringer Ingelheim and Zealand Pharma has demonstrated superior efficacy with survodutide (also known as BI 456906) versus placebo in people with overweight or obesity without type 2 diabetes after 46 weeks of treatment. The findings were presented at the 2023 American Diabetes Association’s 83rd Scientific Sessions in San Diego, CA, US.
Pharmaceutical Technology
JULY 4, 2023
Moderna has filed a regulatory application seeking EMA approval for its modified Covid-19 vaccine targeting the XBB.1.5 sub-variant.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharma Times
JULY 4, 2023
Collaboration aims to revitalise two empty sites on the South Bank of London by providing several laboratories - News - PharmaTimes

Pharmaceutical Technology
JULY 4, 2023
How can you reduce drop-out, save time and resources, and maintain participant engagement throughout the entirety of your trial?

BioPharma Reporter
JULY 4, 2023
Sosei Heptares has dosed the first subject in a phase 1 trial evaluating HTLâ149, a first-in-class GPR52 agonist, which represents a novel mechanism of action for the treatment of schizophrenia and related neurological diseases.
Pharmaceutical Technology
JULY 4, 2023
NorthX Biologics has acquired Valneva’s clinical trial manufacturing unit in Stockholm, Sweden to bolster its expertise.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
pharmaphorum
JULY 4, 2023
Providing access to quality vaccines in low- and middle-income countries Mike.
Pharmaceutical Technology
JULY 4, 2023
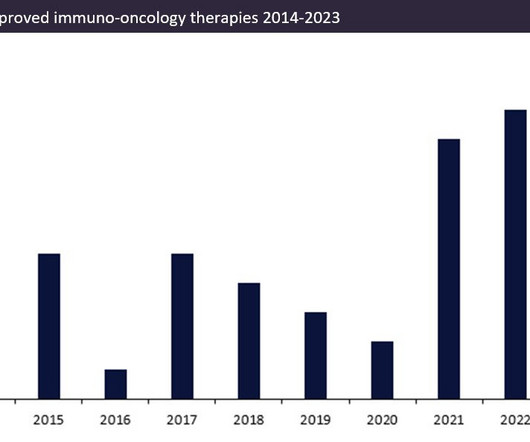
Over the last ten years, the number of approved innovator immuno-oncology therapies (immunotherapies) has increased.
pharmaphorum
JULY 4, 2023
Janssen celebrates positive results for JNJ-2113A in psoriasis trial Eloise.
Pharmaceutical Technology
JULY 4, 2023
The venous thromboembolism (VTE) market is expected to grow from $3.6bn to $4.6bn globally, according to GlobalData.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
BioPharma Reporter
JULY 4, 2023
Camena Bioscience, a synthetic biology company providing genes to the pharmaceutical and biotechnology industries, has closed a $10 million Series A financing round, led by Mercia.

Pharmaceutical Technology
JULY 4, 2023
Tonix Pharmaceuticals and Tonix Medicines have acquired two migraine products from Upsher-Smith Laboratories.

pharmaphorum
JULY 4, 2023
Psychedelics and mental health: The time is now? Nicole.

Pharmaceutical Technology
JULY 4, 2023
The spin-off will create a new global Phase I-IV CRO, patient access and technology solutions.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
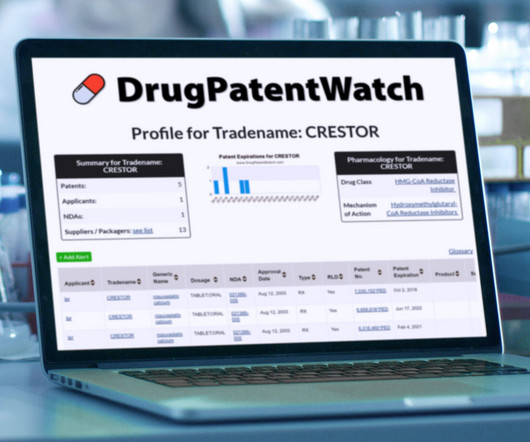
Drug Patent Watch
JULY 4, 2023
[
Drug Discovery World
JULY 4, 2023
Shares in AstraZeneca fell by 6% on July 3 when the company published the first results from its Phase III trial for datopotamab deruxtecan (Dato-DXd) and continued to fall throughout the day, though there was some recovery on July 4. It was reported that the therapy demonstrated statistically significant improvement in progression-free survival vs docetaxel in previously treated locally advanced or metastatic non-small cell lung cancer (NSCLC).
BioPharma Reporter
JULY 4, 2023
Enterome, a clinical-stage company developing first-in-class immunomodulatory drugs for solid and liquid malignancies and inflammatory diseases, has announced that the first patient has been dosed in its phase 1/2 trial evaluating EO4010.
Drug Discovery World
JULY 4, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation to Eli Lilly’s mirikizumab for moderately to severely active ulcerative colitis. The drug is approved in adult patients who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic treatment.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
JULY 4, 2023
Retaining the human quality of healthcare in DTx Mike.

Drug Discovery World
JULY 4, 2023
Vesalius Biocapital, a life sciences venture capital investor, has closed its fourth fund, Vesalius Biocapital IV, securing over €95 million of commitments. The company plans to invest in first and best-in-class European life science companies in drug development and digital health. The goal is to build a portfolio of 10-15 companies, providing capital and the Vesalius team’s experience and expertise to support their development.

pharmaphorum
JULY 4, 2023
So it begins: next Humira biosimilars launch in US Nicole.

Drug Discovery World
JULY 4, 2023
Download this In Focus eReport to gain a full understanding of the current market for mRNA vaccines and therapeutics and the areas of greatest commercial potential for the future. The eReport includes: In-depth analysis of regional markets An overview of the likely challenges and opportunities The companies taking the lead on mRNA development Insights on the future of nucleic acid-based drug discovery and development The therapeutic areas where mRNA could make the biggest impact The potential ro

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content