Moderna's first FDA clearance brings the US a second coronavirus vaccine
Bio Pharma Dive
DECEMBER 18, 2020
The FDA's emergency authorization of the biotech’s shot bolsters an immunization campaign in the U.S. that’s just beginning.

Bio Pharma Dive
DECEMBER 18, 2020
The FDA's emergency authorization of the biotech’s shot bolsters an immunization campaign in the U.S. that’s just beginning.

World of DTC Marketing
DECEMBER 18, 2020
SUMMARY: The “goodwill” that many believe was gained from the development of a Covid-19 may be more of a wish than a reality. Pharma companies are still too focused on profits while people continue to rely on prescription drugs to compensate for unhealthy lifestyles. The headline and byline in a Times editorial said it all “Don’t Fall for Big Pharma’s Savior Act, Heroic work went into the development of the coronavirus vaccines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
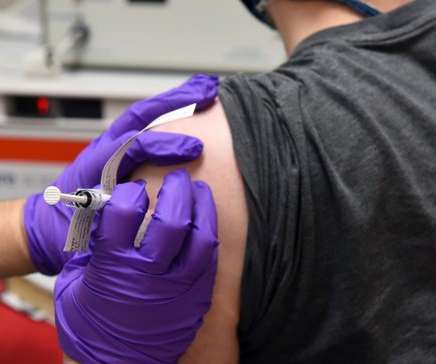
Bio Pharma Dive
DECEMBER 18, 2020
Several thousand doses were returned and replaced after getting too cold. Pfizer and the federal government, meanwhile, don't appear to be on the same page for the next wave of shipments.
pharmaphorum
DECEMBER 18, 2020
Researchers in the UK have developed a chatbot-enabled website that can train people to detect and counter misinformation on COVID-19 and other topics, using techniques from some of philosophy’s greatest critical thinkers. Earlier this year, the World Health organisation (WHO) said the world was dealing with an “infodemic” that is accompanying the pandemic and it is hard for the general public to recognise the “grey area of” misinformation.

Bio Pharma Dive
DECEMBER 18, 2020
How can pharma marketers overcome today's cost-constrained, competitive environment?

NY Times
DECEMBER 18, 2020
The Food and Drug Administration authorized a second coronavirus vaccine for emergency use, clearing the way for millions more Americans to be immunized next week.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
pharmaphorum
DECEMBER 18, 2020
Amgen has filed its groundbreaking KRAS inhibiting drug sotorasib with the FDA for a group of lung cancer patients with an aggressive form of the disease. The drug was the first targeted at the mutation known as KRAS to show activity in the clinic and provided the biggest talking point at the American Society of Clinical Oncology (ASCO) conference in 2019.

Scienmag
DECEMBER 18, 2020
A future of fewer Christmas trees and other conifers Credit: Joseph Stewart, USGS/UC Davis In the aftermath of megafires that devastated forests of the western United States, attention turns to whether forests will regenerate on their own or not. Forest managers can now look to a newly enhanced, predictive mapping tool to learn where forests […].

Pharma Times
DECEMBER 18, 2020
British pharma company also announces strategic collaboration agreement
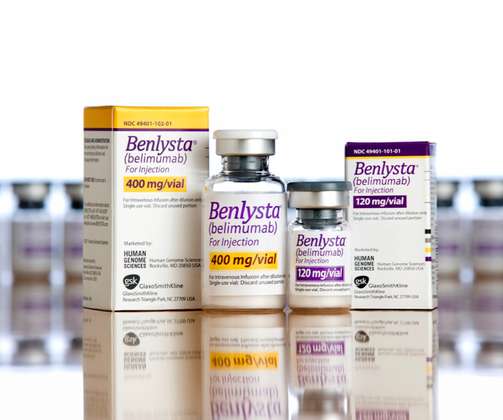
pharmaphorum
DECEMBER 18, 2020
GlaxoSmithKline’s Benlysta has been on the market for almost a decade, but it still has some tricks up its sleeve – it’s just become the first and only FDA-approved treatment for lupus nephritis. The US regulator has cleared both intravenous and subcutaneous formulations of Benlysta (belimumab) for the new indication, extending the use of the drug beyond its earlier label covering the treatment of active systemic lupus erythematosus (SLE) in combination with other medicines.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

NY Times
DECEMBER 18, 2020
Four people so far have had allergic reactions after getting the Pfizer-BioNTech vaccine. Experts say that shouldn’t deter most people from getting a jab.
Pharmacy Checkers
DECEMBER 18, 2020
In its lawsuit to stop wholesale drug importation programs that could help lower U.S. drug prices, the Pharmaceutical Researchers and Manufacturers of America (PhRMA) may be stepping on its toes in helping to allow more personal drug importation. Last month, along with co-plaintiffs Partnership for Safe Medicines (PSM) and Council for Affordable Health Coverage (“CAHC”), PhRMA sued the Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) to invalidate the cert

Pharma Times
DECEMBER 18, 2020
SN14 will be advanced through further development and clinical trials

pharmaphorum
DECEMBER 18, 2020
The FDA is looking to quickly approve Moderna’s COVID-19 vaccine after it was unanimously backed by a panel of experts. Yesterday’s advisory panel meeting voted 20-0 in favour of approving Moderna’s vaccine and although the vote is non-binding, you could probably bet your house on the FDA backing the shot as it rarely goes against the advice of its experts after such strong backing.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

VirTrial
DECEMBER 18, 2020
Technology has infiltrated the clinical research space and is transforming the way research gets done. Predominant solutions across the healthcare and bioscience industries include electronic data capture (EDC), IVRS/registration systems, clinical trial management software (CTMS), e-regulatory tools (i.e., for trial master files, site binders), trial management portals, and virtual trial platforms.

pharmaphorum
DECEMBER 18, 2020
COVID-19 has laid the UK’s health inequalities bare and created an imperative for the NHS to develop a step-change in how it cares for diverse and marginalised communities. Health inequality is the “greatest societal challenge of our time”, according to a briefing document calling for a system-wide solution. The report was published by the Association of the British Pharmaceutical Industry (ABPI) and the NHS Confederation as part of the NHS Reset project, which has been working to set a post-pan

Pharma Times
DECEMBER 18, 2020
Specialist help will be available at 69 sites across the country

pharmaphorum
DECEMBER 18, 2020
By listening to the people who are navigating this new landscape everyday. Integrating personal experiences and patient-reported data, Health Union recently hosted a one-day virtual event entitled The COVID-19 Effect: How Pharma Can Adapt to the Evolving Patient Experience , featuring real-time conversations between people living with chronic conditions and industry experts.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

The Pharma Data
DECEMBER 18, 2020
Action Follows Thorough Evaluation of Available Safety, Effectiveness, and Manufacturing Quality Information by FDA Career Scientists, Input from Independent Experts. SILVER SPRING, Md. , Dec. 18, 2020 /PRNewswire/ — Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the second vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
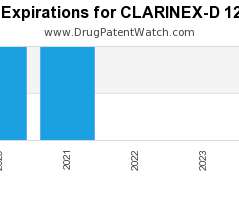
Drug Patent Watch
DECEMBER 18, 2020
Annual Drug Patent Expirations for CLARINEX-D+12+HOUR Clarinex-d 12 Hour is a drug marketed by Merck Sharp Dohme and is included in one NDA. It is available from one supplier. There…. The post New patent expiration for Merck Sharp drug CLARINEX-D 12 HOUR appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
DECEMBER 18, 2020
FDA Approves Orgovyx (relugolix) as the First Oral Gonadotropin-Releasing Hormone (GnRH) Receptor Antagonist for Advanced Prostate Cancer. BASEL, Switzerland, Dec. 18, 2020 (GLOBE NEWSWIRE) — Myovant Sciences (NYSE: MYOV), a healthcare company focused on redefining care for women and for men, today announced that the U.S. Food and Drug Administration (FDA) has approved Orgovyx (relugolix) for the treatment of adult patients with advanced prostate cancer.
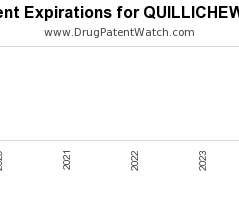
Drug Patent Watch
DECEMBER 18, 2020
Annual Drug Patent Expirations for QUILLICHEW+ER Quillichew Er is a drug marketed by Nextwave Pharms and is included in one NDA. It is available from two suppliers. There are six…. The post New patent for Nextwave Pharms drug QUILLICHEW ER appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

The Pharma Data
DECEMBER 18, 2020
18 December 2020 — The US Food and Drug Administration (FDA) has requested further clarifying analyses of clinical data, to complete its review of the New Drug Application (NDA) for roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor for patients with anemia of chronic kidney disease (CKD). AstraZeneca and FibroGen, Inc.
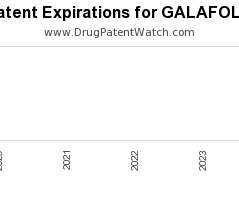
Drug Patent Watch
DECEMBER 18, 2020
Annual Drug Patent Expirations for GALAFOLD Galafold is a drug marketed by Amicus Theraps Us and is included in one NDA. There are sixteen patents protecting this drug. This drug…. The post New patent for Amicus Theraps drug GALAFOLD appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
DECEMBER 18, 2020
News. Professional. Soft Contact Lenses Safe in Children. FRIDAY, Dec. 18, 2020 — Children and young teens can safely wear soft contact lenses (SCLs), according to a study recently published in Ophthalmic & Physiological Optics. In an effort to assess the safety of SCLs, Robin L. Chalmers, a clinical trial consultant in Atlanta, and colleagues retrospectively reviewed charts from 963 children (8 to 12 years old; 782 patients in seven U.S. eye care clinics and 181 children from two inte
Drug Patent Watch
DECEMBER 18, 2020
Annual Drug Patent Expirations for DELSTRIGO Delstrigo is a drug marketed by Msd Merck Co and is included in one NDA. It is available from one supplier. There are two…. The post New patent for Msd Merck drug DELSTRIGO appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

The Pharma Data
DECEMBER 18, 2020
FRIDAY, Dec. 18, 2020 — Many Americans are working at home or attending school virtually during the COVID-19 pandemic, leading to increased use of home heating and its potential risks, an expert says. Heating sources can pose electrical hazards and fire dangers, noted Purnima Unni, manager of the pediatric trauma injury prevention program at Monroe Carell Jr.
Scienmag
DECEMBER 18, 2020
Researchers take a closer look at the genomes of microbial communities in the human mouth Credit: Photo credit: Jessica Mark Welch, Marine Biological Laboratory. Bacteria often show very strong biogeography – some bacteria are abundant in specific locations while absent from others – leading to major questions when applying microbiology to therapeutics or probiotics: how […].
The Pharma Data
DECEMBER 18, 2020
News. Professional. U.S. Indigenous Communities Start Receiving COVID-19 Vaccines. FRIDAY, Dec. 18, 2020 — Indigenous communities in the United States have started receiving COVID-19 vaccines from federal and state agencies. The initial focus of the federal government’s Indian Health Service is to vaccinate health care workers at sovereign Indigenous nation clinics nationwide and urban clinics that provide care for off-reservation Native Americans, the Associated Press reported.
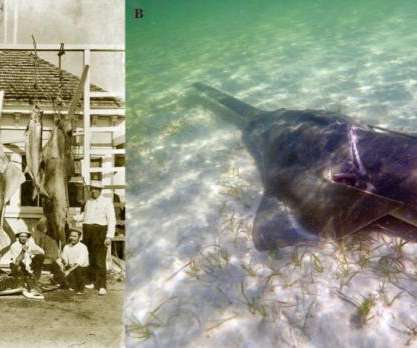
Scienmag
DECEMBER 18, 2020
The findings have important implications to better protect this endangered species Credit: see above MIAMI–A new collaborative study lead by scientists at the University of Miami (UM) Rosenstiel School of Marine and Atmospheric Science and the National Oceanic and Atmospheric Administration (NOAA) found evidence of growing numbers of critically endangered smalltooth sawfish within coastal waters […].

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content