Lyfgenia and Casgevy Become First FDA-Approved Gene Therapies for Sickle Cell Disease
XTalks
DECEMBER 13, 2023
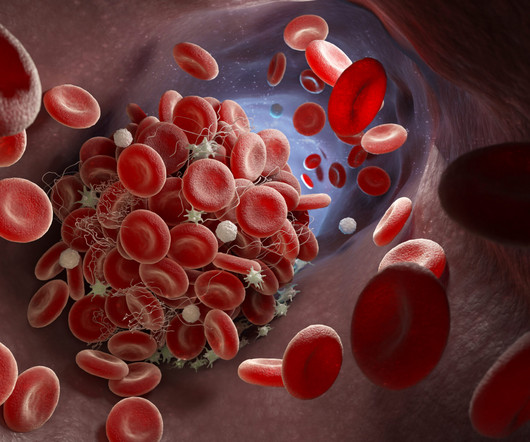
The disease occurs due to a point mutation in the hemoglobin beta globin ( HBB ) gene that codes for one of the proteins that make up hemoglobin, the oxygen carrier in red blood cells. Casgevy’s approval by the FDA is momentous: it is the first CRISPR-based gene-editing therapy to be approved in the US.


















Let's personalize your content