Market cap downturn hits over half of top 20 biopharmaceutical companies in Q1 2023
Pharmaceutical Technology
MAY 16, 2023
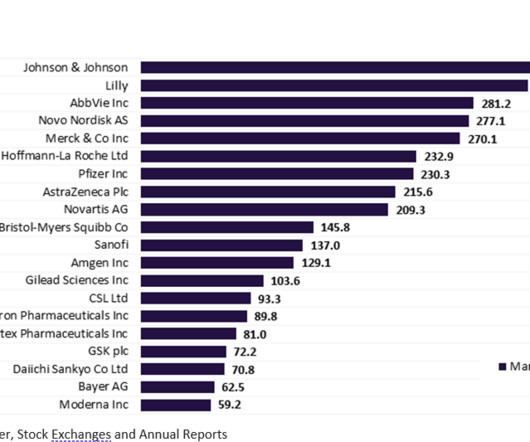
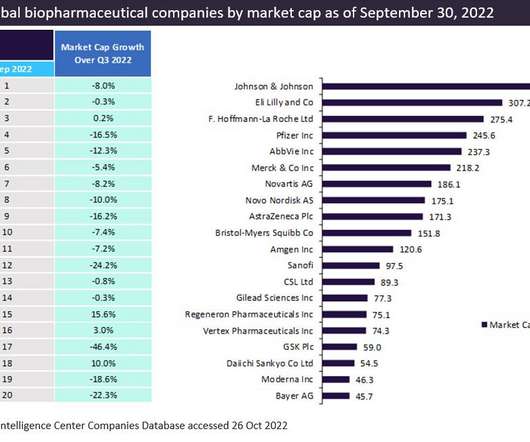
More than half of the top 20 global biopharmaceutical companies saw a fall in market capitalisation over Q1 2023. decline in total aggregate market capitalisation from $3.61 Bayer reported the highest market capitalisation growth of 23.1% Sanofi and Regeneron’s market capitalisation grew by 12.4% This resulted in a 3.4%
























Let's personalize your content