Bristol Myers taps startup to boost CAR-T production
Bio Pharma Dive
APRIL 22, 2024
A partnership with Cellares, worth up to $380 million, is meant to help Bristol Myers speed and scale manufacture of the complex cellular treatments.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Bio Pharma Dive
APRIL 22, 2024
A partnership with Cellares, worth up to $380 million, is meant to help Bristol Myers speed and scale manufacture of the complex cellular treatments.

AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 3, 2024
The Drugs Consultative Committee (DCC), the advisory committee of the Central government to advise the Central and state governments on matters that require uniform implementation of drug laws across the country, has recommended to all the States to set a deadline for the manufacturing companies to upload all the formulation details in the Sugam portal, (..)
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
APRIL 11, 2024
With specialised production facilities for HPAPIs in great demand, what are the key challenges in manufacturing?

Pharmaceutical Technology
MARCH 6, 2023
Incannex Healthcare has collaborated with New Jersey-based pharma company Catalent for the development and manufacturing of a cGMP-grade psilocybin drug product for clinical trials and potential commercial use. It will also be potentially used for wider commercial use or supply as a cGMP pharmaceutical-grade product.
Advertisement
As demand for advanced therapies increases, so does the need for more specialized supply chain support, as these products have strict transportation and handling requirements. It is crucial to ensure supply chain risks are mitigated and have proactive strategies in place to address unforeseen challenges before they become an issue.

Pharma Mirror
MARCH 29, 2022
NIEL, BELGIUM, eTheRNA Manufacturing, a specialist RNA process developer and manufacturing member of the Belgian eTheRNA group, is introducing a new Lipid Nanoparticle (LNP) formulation development and production service to support the discovery and early pre-clinical development of RNA-based therapeutics and vaccines.

Pharmaceutical Technology
MARCH 13, 2024
As the demand for medical devices continues to rise, manufacturers are increasingly turning to automation during the assembly process to get their products to market faster. But how can the risks involved be mitigated?

Pharmaceutical Technology
JULY 15, 2022
Insulin prices made the headlines again as California governor Gavin Newsom announced plans on 7 July for the state to manufacture low-cost insulin. The state plans to work directly with a contract manufacturing organization (CMO) to manufacture low-cost insulin. In-house manufacturing the norm.

Pharmaceutical Technology
FEBRUARY 21, 2024
Contract development and manufacturing organisation Genezen has announced plans to license CSL’s Cytegrity lentivirus production system.
Pharmaceutical Technology
APRIL 13, 2023
The initial agreement between Dyadic and Rubic involved discovery, development, manufacturing, and distribution of Covid-19 vaccines by transferring and licensing of C1 platform technology. The now expanded agreement will enable Rubic to explore other therapeutic avenues in both the human and animal health product markets.

Pharmaceutical Technology
JUNE 7, 2023
Shanghai Fosun Pharmaceutical (Group) is to collaborate with the International Finance Corporation (IFC) to construct a new pharmaceutical production facility and distribution hub near Abidjan, Côte d’Ivoire. The manufacturing site will be completed in three phases and will produce anti-bacterial medicines and anti-malarial drugs.

Roots Analysis
APRIL 15, 2024
Further, the manufacturing of biologics fill finish is a highly complex and cost-intensive process. Notable examples of fill finish manufacturing companies equipped with SA25 aseptic filling workstation include ( in alphabetical order ) Emergent Bioservices, PCI Pharma Services, Singota Solutions and WuXi Biologics.

Pharmaceutical Technology
MARCH 10, 2023
On 10 March, the National Health Service Blood and Transplant (NHSBT) opened a new Clinical Biotechnology Centre (CBC) with the aim of improving the UK’s ability to develop and manufacture cell and gene therapies. This is important due to the UK’s currently limited short-scale manufacturing capacity.

Pharmaceutical Technology
MARCH 31, 2023
Moderna has finalised an agreement with the government of the Republic of Kenya to establish an mRNA manufacturing facility in the country. The company is also committed to establishing mRNA manufacturing facilities in Australia, Canada, the US and the UK.

Fierce Pharma
MARCH 1, 2024
Moderna is laying off employees within its manufacturing unit, with the move tied to a resizing of its COVID production work. | Moderna is laying off some employees within its manufacturing unit after shaving COVID production costs.

Pharmaceutical Technology
MARCH 13, 2024
In T-cell manufacturing, the historical issue of maintaining the quality of materials when scaling up production is changing.

Pharmaceutical Commerce
MARCH 27, 2024
In this Pharmaceutical Commerce video interview, Barry Heavey, Life Sciences Supply Chain Lead, Accenture, discusses how companies can leverage digital tools like artificial intelligence and machine learning to optimize manufacturing processes and ensure efficient production.

Pharmaceutical Technology
DECEMBER 4, 2023
GC Biopharma has concluded the construction activities at an mRNA manufacturing plant at its vaccine production site in South Korea.

Fierce Pharma
FEBRUARY 22, 2024
To get a pulse on Moderna following its “year of transition” in 2023, look no further than the company’s manufacturing operations. | To get a pulse on Moderna following its “year of transition” in 2023, look no further than the company’s manufacturing operations.
Pharma Mirror
SEPTEMBER 1, 2023
North Charleston, SC: Pharmaceutical sterilization and cleaning equipment manufacturer Belimed Life Science, North Charleston, SC, has unveiled custom wash rack design and manufacturing services.

Pharmaceutical Technology
NOVEMBER 14, 2022
BioNTech’s Singapore affiliate BioNTech Pharmaceuticals Asia Pacific has signed an agreement with Novartis Singapore Pharmaceutical Manufacturing to acquire a GMP-certified manufacturing site in the country. . It also has the potential for extension into the manufacturing of other drug classes such as cell therapies. .

Bio Pharma Dive
APRIL 26, 2023
Accessing the Illinois facility will expand Bristol Myers’ supply of viral vectors following manufacturing struggles with CAR-T drugs Abecma and Breyanzi.

Pharmaceutical Technology
JANUARY 12, 2024
Cereno Scientific has announced a partnership with CordenPharma to scale up manufacture of its drug candidate CS1.
Pharmaceutical Technology
AUGUST 9, 2023
GenScript Biotech has expanded the peptide production capabilities at its new life-sciences manufacturing facility located in Zhenjiang.
Pharmaceutical Technology
NOVEMBER 22, 2022
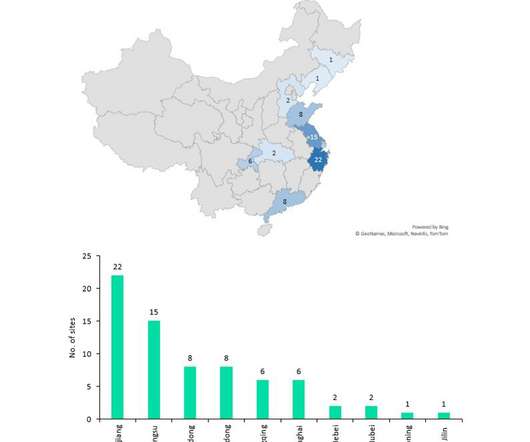
Despite China producing a significant proportion of the world’s API supply (mostly small molecule), it manufactures relatively few biosimilar and innovator drugs and no cell and gene therapies for the western markets of Europe and the US despite investments and an increasing number of startups to improve innovative manufacture.

BioSpace
SEPTEMBER 26, 2023
The COVID-19 pandemic highlighted a need for local production of vaccines. Now, German pharma company BioNTech has said it will start manufacturing vaccines in Africa.
Pharmaceutical Technology
APRIL 4, 2023
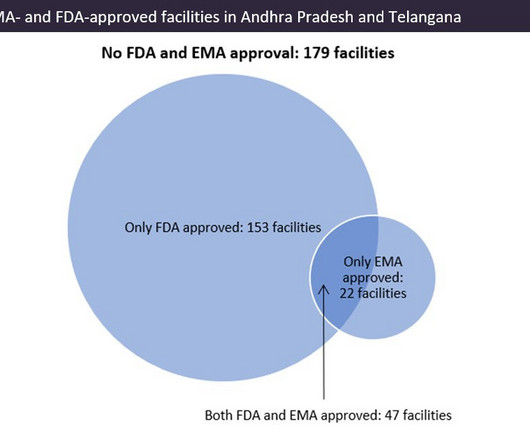
Indian pharma manufacturing continues to be the backbone of drug supplies worldwide, and GlobalData analysis suggests US overreliance on the country for generic drug supply. Pharma manufacturing facilities in Andhra Pradesh and Telangana accounted for 22.5% © GlobalData. © GlobalData. ©GlobalData.

Pharmaceutical Technology
MARCH 13, 2023
Cytovance Biologics has entered a collaboration deal with Phenotypeca to enhance the development of saccharomyces cerevisiae strain for biopharmaceutical manufacturing. The partnership is expected to expedite the development of high-performance yeast strains and improve the biologics production efficiency for biopharma clients.

Pharma Mirror
JANUARY 24, 2023
Science-based innovation for manufacturing high quality cells challenges dogma and terminology Cell and gene therapies are dominating the world of drug development. billion by 2026, up from $186 billion in 2019 according to the Evaluate Pharma report.

Fierce Pharma
APRIL 18, 2024
History is coming full circle as induced pluripotent stem (iPS) cell-derived cell therapy maker Shinobi Therapeutics links up with Panasonic and Japan’s Kyoto University, where the first mouse iPS | Shinobi Therapeutics has entered an accord with electronics powerhouse Panasonic and Kyoto University in a bid to develop a new closed-system manufacturing (..)

pharmaphorum
AUGUST 12, 2022
The COVID-19 pandemic led to skyrocketing demand for some products while simultaneously constraining supplies due to logistical breakdowns and protectionist practices. The logic of outsourcing production to other countries, primarily China, suddenly looked dubious as Western countries struggled to import the drugs they needed.

XTalks
SEPTEMBER 1, 2023
GlaxoSmithKline (GSK) has been pumping large sums of cash into expanding its vaccine manufacturing operations and today, it announced an investment of €250 ($272 million) into building a new unit for freeze-drying vaccines at its Wavre campus in Belgium. Both vaccines will be manufactured at the new unit in Belgium. billion ($2.1

Pharmaceutical Technology
FEBRUARY 8, 2023
Genenta Science and AGC Biologics have signed a development and manufacturing service agreement (MSA). Under the deal, AGC Biologics will be responsible for manufacturing the cell therapy lentivirus-based product for the ongoing clinical programmes of Genenta Science.
Pharmaceutical Technology
JUNE 23, 2022
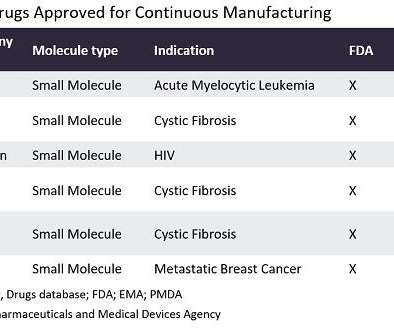
In the last year, well-known contract manufacturing organisations (CMOs) such as Agilent Technologies (Santa Clara, California) and Abzena (Cambridge, UK) have expanded their continuous manufacturing capabilities. US Congress is also making substantial investments to improve the continuous manufacturing of pharmaceuticals.

Pharmaceutical Technology
SEPTEMBER 26, 2023
To optimise autoinjector combination product development, it is crucial for pharma and biotech to partner with device companies that have in-house control over the whole device manufacturing process.

Fierce Pharma
NOVEMBER 22, 2023
Unable to scale up its manufacturing fast enough to meet the spiraling demand for its GLP-1 weight loss products, Novo Nordisk is employing a new strategy—reducing production of diabetes drug Victo | Unable to scale up its manufacturing fast enough to meet the spiraling demand for its GLP-1 weight loss products, Novo Nordisk is employing a new strategy—reducing (..)
pharmaphorum
FEBRUARY 9, 2024
Learn how implementing a SMART approach in stem cell manufacturing can lead to improved efficiency and cost reduction for the production of mesenchymal stem cells (MSCs) used in the treatment of osteoarthritic and autoimmune conditions.

Fierce Pharma
JANUARY 22, 2024
Eli Lilly’s beleaguered manufacturing site in Branchburg, New Jersey, is back in the U.S. Following a July inspection, the FDA has uncovered eight new production deficiencies at Eli Lilly's Branchburg production plant. Food and Drug Administration’s crosshairs, Reuters first reported.

Pharmaceutical Technology
NOVEMBER 1, 2022
FEATURED COMPANIES COMMENDED : • Innovation • Product Launch • Safety VIEW PROFILE COMMENDED : • Business Expansion • Innovation • Investments VIEW PROFILE COMMENDED: • Social VIEW PROFILE. What are Product Launches? The Product Launches category recognises companies that have launched notable new products or services into the market.

Pharma Mirror
DECEMBER 14, 2020
The future of pharmaceutical manufacturing is mobile. has led to the proliferation of mobile IT solutions for manufacturing, including pharmaceuticals. The post Best Features of Mobile Workstations in Pharmaceutical Production appeared first on Pharma Mirror Magazine. Advances in cloud computing and the onset of Industry 4.0
Bio Pharma Dive
AUGUST 24, 2023
Bristol Myers Squibb is among those backing the startup, which claims the manufacturing capacity at its New Jersey plant can surpass that of conventional CDMO facilities.

Pharmaceutical Technology
JUNE 13, 2023
Novo Nordisk has unveiled plans to make a DKr15.9bn ($2.29bn) investment , starting from 2023, to expand its manufacturing facilities in Hillerød, Denmark. The investment will be used for expanding its existing Danish active pharmaceutical ingredient (API) production facility to progress its future serious and chronic disease portfolio.

XTalks
MAY 27, 2021
To help boost production of its COVID-19 vaccine, Moderna has enlisted the services of Samsung Biologics , a contract development and manufacturing organization (CDMO) based in South Korea. The vaccine doses are intended for countries outside the US, with manufacturing planned to commence in the third quarter of 2021.

Pharmaceutical Technology
MAY 23, 2023
Croda Pharma has entered a strategic collaboration deal with Botanical Solution Inc (BSI) to expedite the production of sustainable pharmaceutical-grade vaccine adjuvant QS-21. The plentiful supply of QS-21 enables the production of next-generation adjuvant systems for new vaccine development.”
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content