WHO grants emergency use listing to SK bioscience’s SKYCovione
Pharmaceutical Technology
JUNE 20, 2023
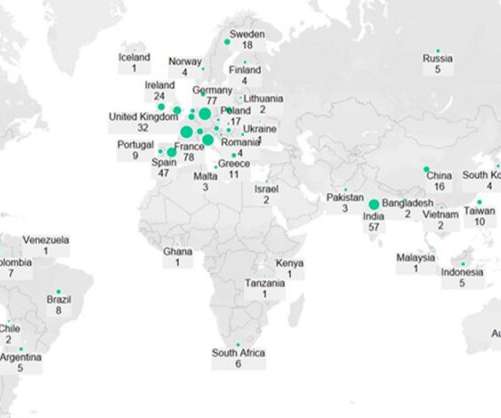
The World Health Organisation (WHO) has granted an emergency use listing (EUL) to SK bioscience’s Covid-19 vaccine, SKYCovione. SKYCovione is a self-assembled nanoparticle vaccine and the 12th Covid-19 vaccine to receive a EUL from the regulator.


































Let's personalize your content