mRNA: A New Era in Biopharmaceutical Contract Manufacturing Market
Roots Analysis
AUGUST 16, 2023
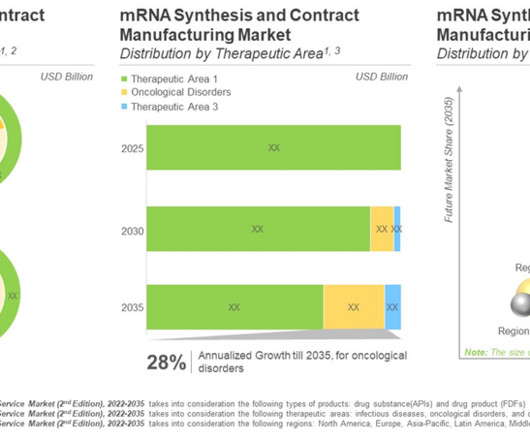
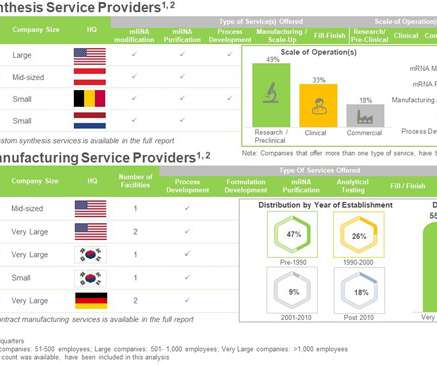
The success of COVID-19 vaccines paved the path for mRNA-based drug products. Given such technical and operational challenges associated with the production of RNA-based products, innovators in the biopharmaceutical industry are increasingly relying on the contract manufacturing service providers to cater to the urgent global demand.











Let's personalize your content