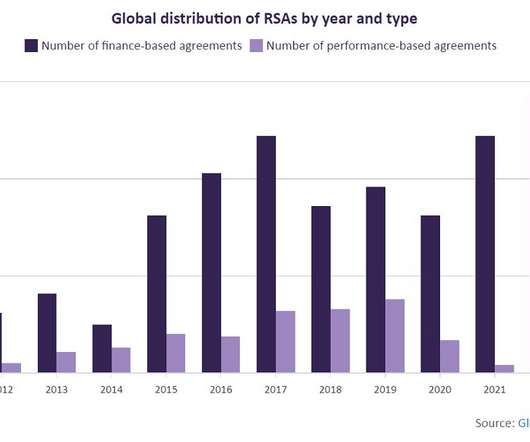
Risk-sharing agreements are growing at a rate of 24%
Pharmaceutical Technology
FEBRUARY 10, 2023
This is not the first treatment to come with a high price tag. Using this categorization, programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors saw the greatest percentage of RSAs granted.




















Let's personalize your content