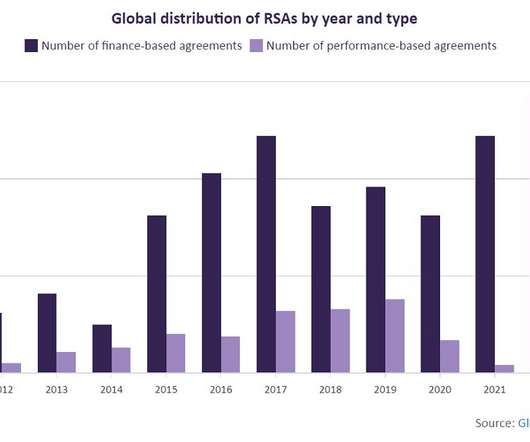
Risk-sharing agreements are growing at a rate of 24%
Pharmaceutical Technology
FEBRUARY 10, 2023
This is not the first treatment to come with a high price tag. Access to oncology medicines varies across countries, and their cost against safety/efficacy is generally perceived as being low, such as in the US. million, according to GlobalData’s Price Intelligence (POLI), making it the most expensive drug in the world.




















Let's personalize your content