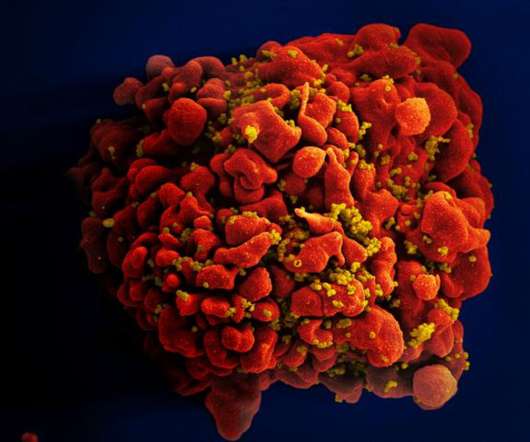
Stem cell therapy ‘cures’ first woman with HIV, say researchers
pharmaphorum
FEBRUARY 16, 2022
. “This is more of a proof-of-concept, than something that can be applicable to the many millions of people living with HIV,” said Dr Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID), which funded the research. 32 mutation into patients.














Let's personalize your content