Kaiser Permanente Joins Late-Stage Trial for Pfizer and BioNTech’s Lead COVID-19 Vaccine Candidate
XTalks
AUGUST 13, 2020
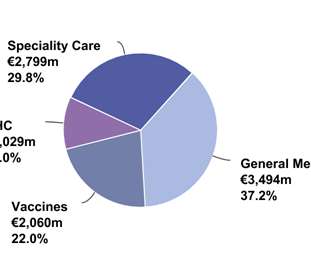
As the rush for a COVID-19 vaccine continues, healthcare services company Kaiser Permanente has joined in on the clinical testing of Pfizer Inc. and BioNTech’s lead vaccine candidate in a Phase III trial being conducted at sites in California and Oregon.


































Let's personalize your content