New COVID-19 Testing Technology + FDA Approves First Drug for HER2-Low Breast Cancer – Xtalks Life Science Podcast Ep. 72
XTalks
AUGUST 10, 2022
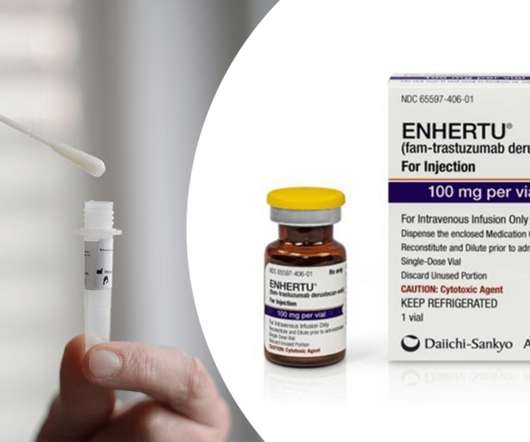
Ayesha also talked about the FDA approval of AstraZeneca and Daiichi Sankyo’s antibody-drug conjugate (ADC) Enhertu (trastuzumab-deruxtecan) for the treatment of patients with unresectable or metastatic HER2-low breast cancer. The approval makes Enhertu the first approved drug for this indication.
































Let's personalize your content