Regeneron’s Antibody Drug Becomes First Approved Treatment for Ebola
XTalks
OCTOBER 16, 2020
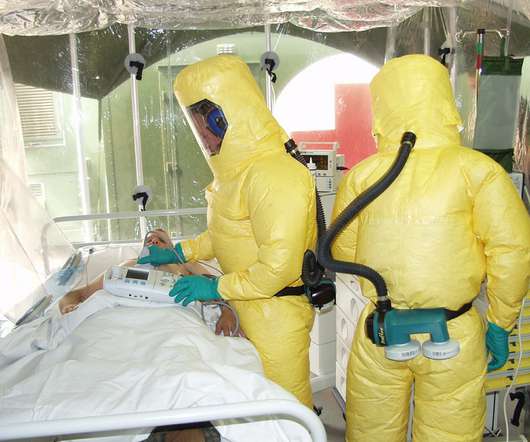
The US Food and Drug Administration (FDA) has approved Inmazeb (atoltivimab, maftivimab and odesivimab-ebgn) as the world’s first treatment for Zaire ebolavirus (Ebola virus) infection in adult and pediatric patients, including newborns of mothers who have tested positive for the virus. Targeted Antiviral Treatment.









































Let's personalize your content