Caris and Merck KGaA link to develop ADCs for cancer treatment
Pharmaceutical Technology
APRIL 5, 2024
Caris Life Sciences has entered a collaboration with Merck KGaA for the development of antibody-drug conjugates (ADCs) for cancer.

Pharmaceutical Technology
APRIL 5, 2024
Caris Life Sciences has entered a collaboration with Merck KGaA for the development of antibody-drug conjugates (ADCs) for cancer.
Pharmaceutical Technology
MAY 8, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Anti-influenza antibody compositions. However, not all innovations are equal and nor do they follow a constant upward trend.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
NOVEMBER 30, 2023
The sizzling antibody-drug conjugate (ADC) field is at the center of another major life sciences deal. | The sizzling antibody-drug conjugate field is at the center of another major life sciences deal. Hoping to redeem itself following the epic Rova-T failure, AbbVie is shelling out $10.1
XTalks
MAY 9, 2024
Genmab, a trailblazer in the field of antibody therapeutics, celebrates this milestone by reflecting on its impressive journey from a small-scale startup to a global leader in cancer treatment and beyond. Since then, innovative science has been at Genmab’s core, as we harness the power of human antibodies to improve the lives of patients.

XTalks
NOVEMBER 2, 2022
In this episode, Ayesha discussed the FDA approval of two new immunotherapies, including Janssen/Johnson & Johnson’s bispecific antibody Tecvayli for the treatment of relapsed or refractory multiple myeloma. The drug is the first bispecific T cell antibody to be approved in the US.

XTalks
FEBRUARY 23, 2022
The team also talked about Eli Lilly’s new COVID-19 monoclonal antibody bebtelovimab that received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) indicated for patients with mild to moderate COVID-19 who are at risk of progressing to severe disease.
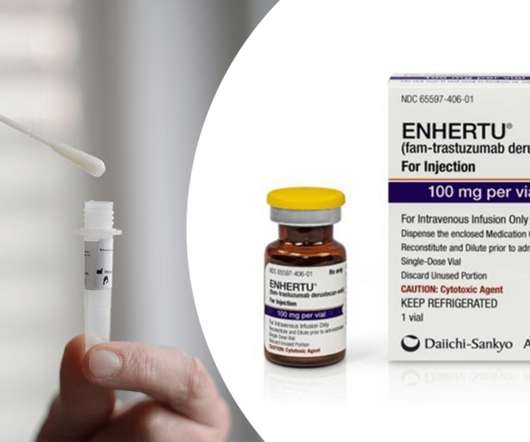
XTalks
AUGUST 10, 2022
Ayesha also talked about the FDA approval of AstraZeneca and Daiichi Sankyo’s antibody-drug conjugate (ADC) Enhertu (trastuzumab-deruxtecan) for the treatment of patients with unresectable or metastatic HER2-low breast cancer. The approval makes Enhertu the first approved drug for this indication.
Let's personalize your content