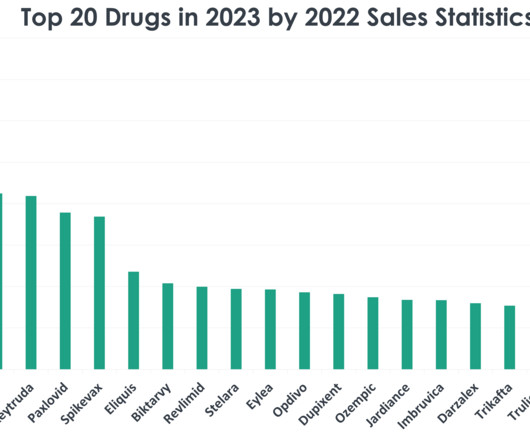
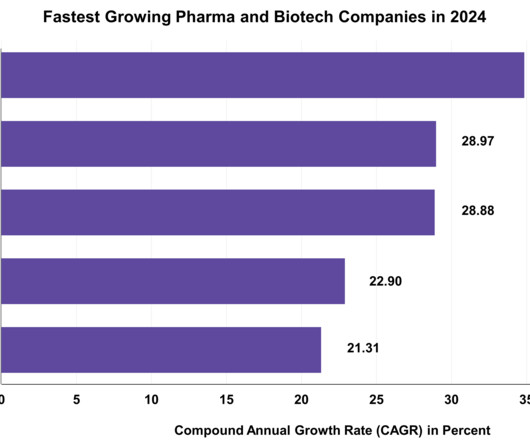
Top 20 Drugs in 2023 by 2022 Sales Statistics
XTalks
DECEMBER 19, 2023
As we approach the end of 2023, a retrospective look at the statistics from 2022 reveals the top 20 drugs dominating retail sales. In this article, we will explore the factors contributing to the success of the top 20 drugs in 2023 by retail sales from the prior year. 1) Comirnaty (COVID-19 Vaccine, mRNA) Sales in 2022: $37.81
































Let's personalize your content