CellCentric Strengthens Leadership Team
Pharma Mirror
APRIL 8, 2022
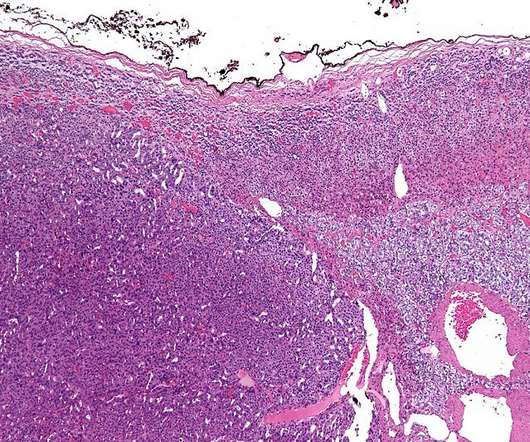
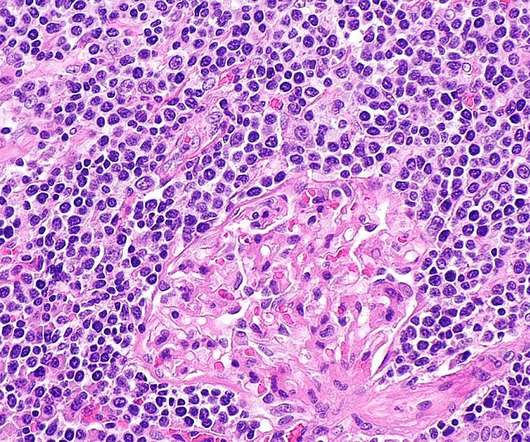
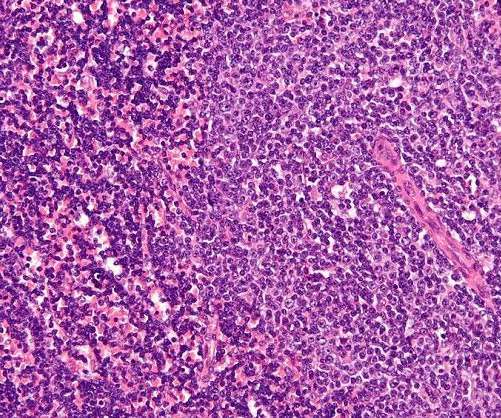
CellCentric is developing inobrodib, an orally bioavailable drug that is transitioning into Phase II clinical trials in multiple indications. Both have stellar experience and track records in oncology research and development.








































Let's personalize your content