Bioavailability Enhancement Technologies
Roots Analysis
FEBRUARY 15, 2023
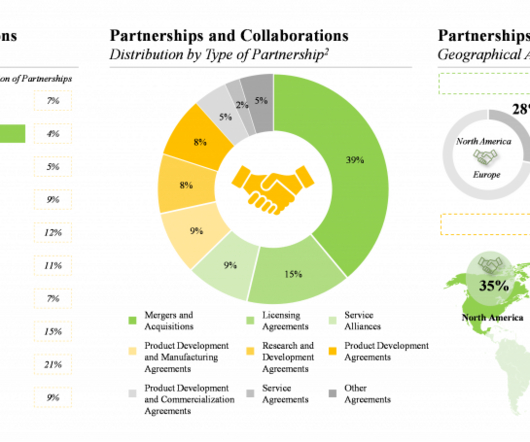
Moreover, it is a well-established fact that the systemic / local absorption and distribution of a therapeutic intervention is directly proportional to its bioavailability. As per our analysis, North America and Europe, with the highest number of technology as well as service providers, have emerged as key hubs.











Let's personalize your content