SEC recommends approval for Zydus Lifesciences’ trivalent influenza vaccine in children
AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 13, 2024
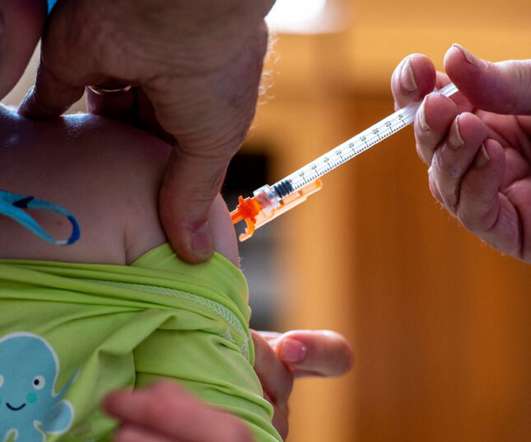
The Subject Expert Committee (SEC), which advises the Central drug regulator regarding clinical trials and approvals of drugs, has recommended approval for additional indication of Zydus Lifesciences’ inactivated trivalent influenza vaccine in children above six months with clinical trial waiver subject to condition.




































Let's personalize your content