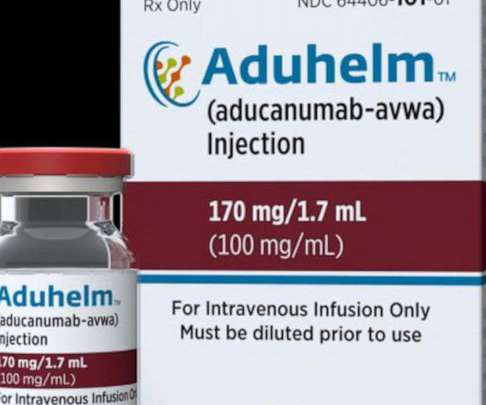
FDA Approves Updated ADUHELM™ Prescribing Information to Emphasize Population Studied in Clinical Trials
The Pharma Data
JULY 8, 2021
The update includes an addition to the Indications and Usage section of the label (Section 1) to emphasize the disease stages studied in the clinical trials, as seen below ( italics to note updated language). The update clarifies the indication by emphasizing information about the disease stages studied in the ADUHELM clinical trials.















































Let's personalize your content