FibroGen and Fortis sign exclusive licence deal for mCRPC drug candidate
Pharmaceutical Technology
MAY 9, 2023
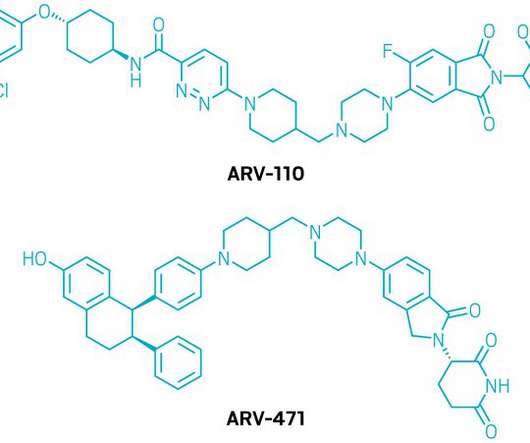
FibroGen has signed an exclusive licence agreement with Fortis Therapeutics for the FOR46 antibody-drug conjugate (ADC) that targets a new epitope on CD46, a protein-coding gene. FOR46 is currently being investigated in a Phase I trial as a potential treatment for metastatic castration-resistant prostate cancer (mCRPC).





































Let's personalize your content