Benefits of Participating in Clinical Trials
Olympian Clinical Research
MARCH 19, 2024
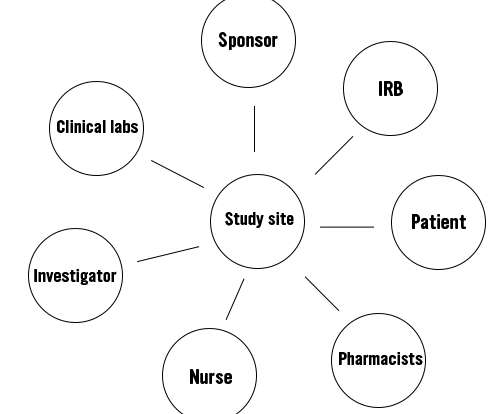
Clinical trials are essential for advancing medical knowledge and developing new treatments that can improve patient care and outcomes. In Tampa Bay, Olympian Clinical Research is at the forefront of this vital work, offering local residents the opportunity to contribute to medical advancements.












































Let's personalize your content