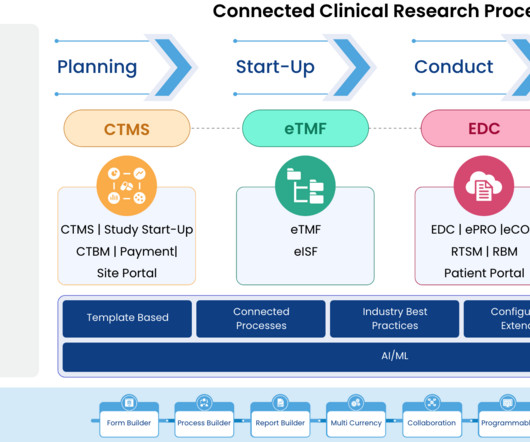
Breaking Barriers: Reasons to Expand Site Cancer Clinical Trial Portfolios
WCG Clinical
JUNE 14, 2024
In this article, we will explore the significant value derived from conducting clinical trials sponsored by pharmaceutical companies for both physicians and hospital systems, with a specific focus on cancer care, and address potential counterarguments.











































Let's personalize your content