Tech innovations that curb complexity, setting clinical trials up for success
Pharmaceutical Technology
OCTOBER 26, 2022
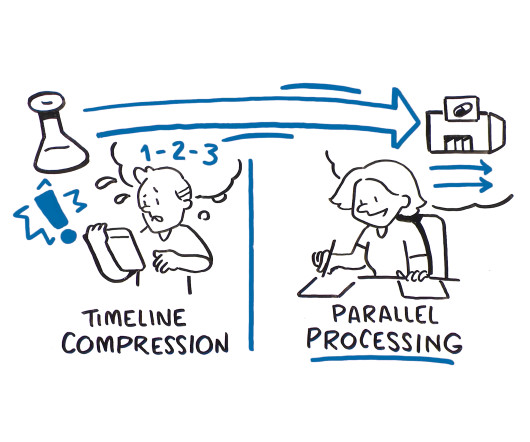
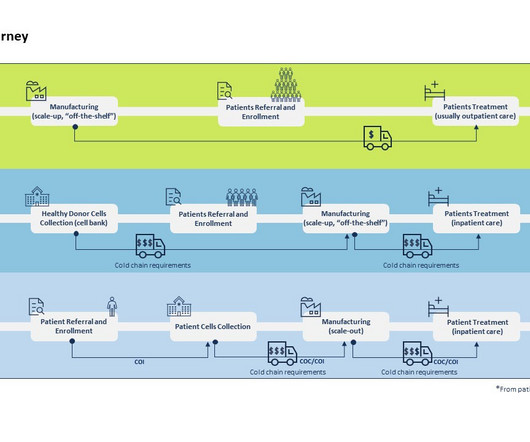
According to Tufts Center for the Study of Drug Development (CSDD), in a typical Phase Ill clinical trial, 119 protocol deviations are implemented on average, involving approximately one-third of all patients participating in that trial. Demand planning drives trial efficiency.



























Let's personalize your content