Biologic Therapeutics Development, Part 2: Regulatory Pathways and Pharmacometric Analysis
Camargo
DECEMBER 13, 2021
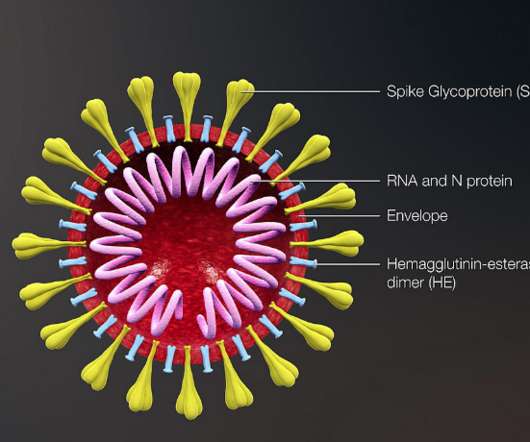
Like with small molecules, clinical trials of biologics are designed to determine pharmacokinetics (PK), pharmacodynamics (PD), safety, and efficacy. The regulations regarding BLAs for therapeutic biological products are included in 21 CFR parts 600 , 601 , and 610. Expanded access to experimental biologics. BLA process (CBER).


































Let's personalize your content