New male contraceptive does not involve hormones
Medical Xpress
NOVEMBER 24, 2022
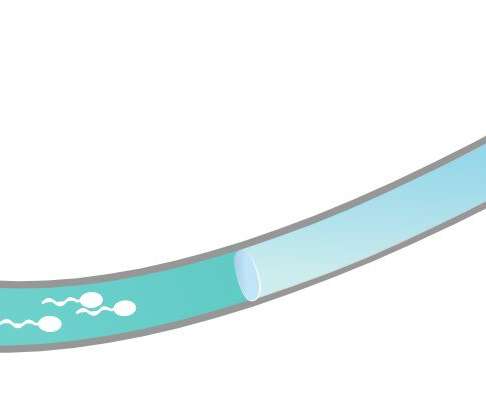
A team of researchers at a company called Contraline has developed a new kind of male contraceptive. Instead of using hormones to disrupt sperm production, the new technique involves placing a hydrogel called ADAM into the vas deferens to prevent sperm from making its way to the urethra.







































Let's personalize your content