FDA approves Covid-19 mAb for emergency use in immunocompromised
Drug Discovery World
MARCH 26, 2024
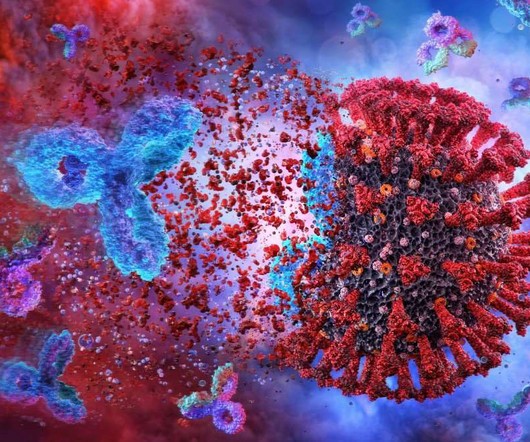
The US Food and Drug Administration (FDA) has granted emergency use authorisation (EUA) to half-life extended monoclonal antibody (mAb) Pemgarda (pemivibart, or VYD222) for the pre-exposure prophylaxis (prevention) of Covid-19.











































Let's personalize your content