What Drives Clinical Trial Costs? A Comprehensive Exploration
Cloudbyz
MAY 11, 2023
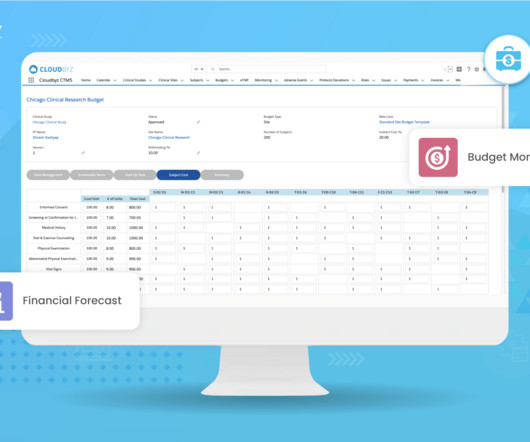
Clinical trials play a vital role in bringing new therapies and treatments to patients, but they can be expensive and resource-intensive endeavors. Understanding the factors driving clinical trial costs is essential for researchers, sponsors, and other stakeholders involved in the drug development process.













































Let's personalize your content