mRNA: A New Era in Biopharmaceutical Contract Manufacturing Market
Roots Analysis
AUGUST 16, 2023
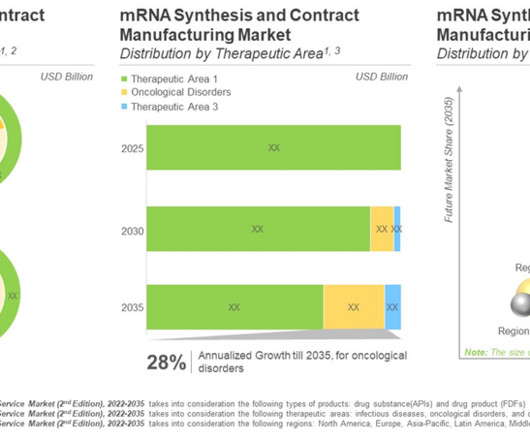
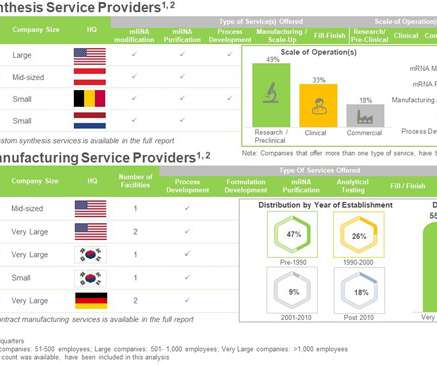
However, synthesis and manufacturing of mRNA is fraught with several challenges as the production of such biomolecules is complex, cost intensive, requires specialized expertise and dedicated equipment. Safety, efficacy and rapid production of mRNA drives the interest in this domain.








































Let's personalize your content