Novel antibodies target ‘dark side’ of influenza virus protein
Drug Discovery World
MARCH 5, 2024
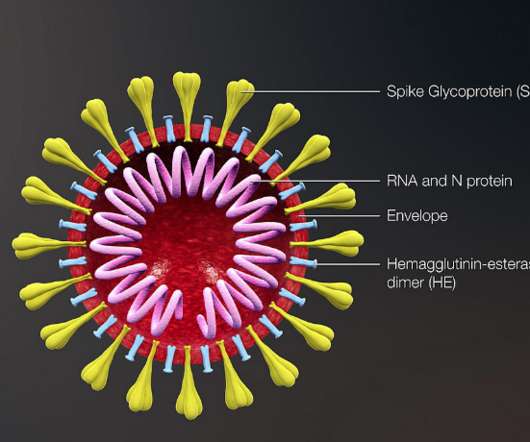
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored ‘dark side’ of the neuraminidase (NA) protein head.





































Let's personalize your content