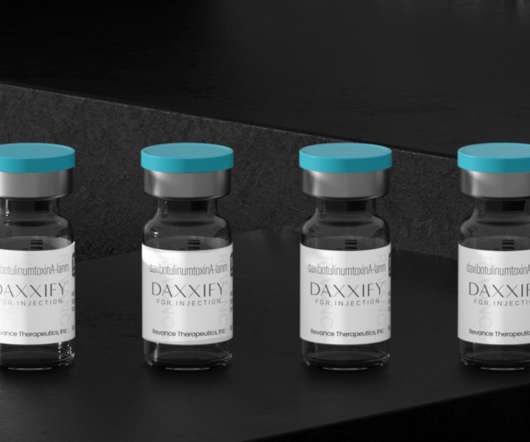
Daxxify, a New Anti-Wrinkle Drug and Botox Competitor is FDA-Approved
XTalks
SEPTEMBER 14, 2022
Daxxify was FDA-approved for similar cosmetic purposes as Botox and other neuromodulators like Dysport and Xeomin. Used for both cosmetic and therapeutic cases, Botox is a US Food and Drug Administration (FDA)-approved injection of botulinum toxin, a neurotoxic protein that can effectively paralyze the facial muscles.






























Let's personalize your content