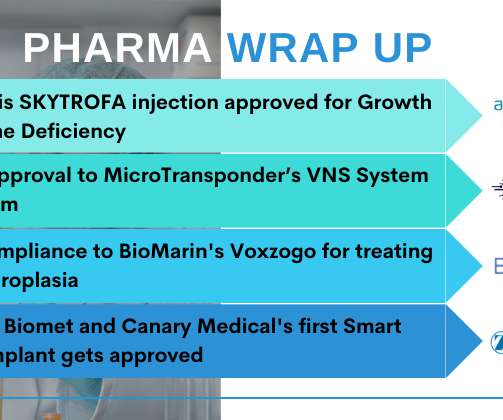
Sanofi snaps up acne vaccine developer Origimm Bio
pharmaphorum
DECEMBER 1, 2021
The French group said that ORI-001, a vaccine based on recombinant proteins from the Cutibacterium acnes (formerly Propionibacterium acnes ) bacterium that is often found in acne lesions, could be the first ever vaccine for the condition. The post Sanofi snaps up acne vaccine developer Origimm Bio appeared first on.








































Let's personalize your content