Telix revives Olaratumab development with positive preclinical data
Pharmaceutical Technology
APRIL 17, 2023
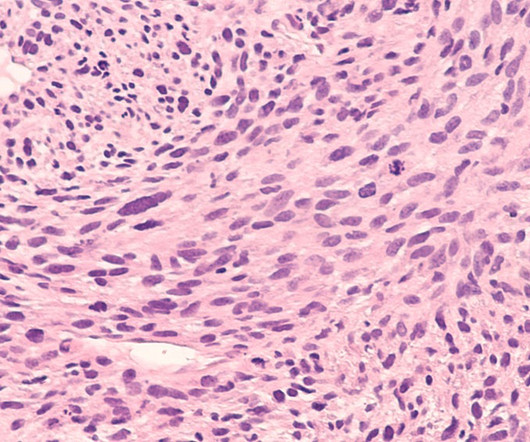
Telix has announced the successful preclinical development of radiolabelled olaratumab, an antibody licensed by Eli Lilly and Company. Olaratumab will be denoted as TLX300 in Telix’s development pipeline. But, following negative Phase III efficacy results, Lilly withdrew the drug from the market.












































Let's personalize your content