Scientists Discover a New, Unexpected Way That Cancer Cells Spread
AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 30, 2023
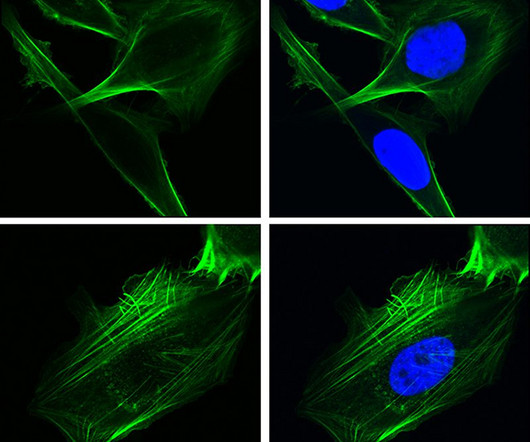
One of the challenges in treating cancer is stopping it from metastasizing, and a new study reveals one of the fundamental mechanisms through which this happens. Now we know about this mechanism, perhaps we can stop it. Key to this newly discovered process is GRP78, and it’s what’s known as a chaperone protein.


































Let's personalize your content